 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Good Laboratory Practices for Waived Testing SitesSurvey Findings from Testing Sites Holding a Certificate of Waiver Under the Clinical Laboratory Improvement Amendments of 1988 and Recommendations for Promoting Quality TestingPrepared by
The material in this report originated in the Coordinating Center for Health Information and Service, Steven L. Solomon, MD, Director; National Center for Health Marketing, Jay M. Bernhardt, PhD, Director; and the Division of Public Health Partnerships, Robert Martin, DrPH, Director. Corresponding author: Devery Howerton, PhD, National Center for Health Marketing, Coordinating Center for Health Information and Service; 4770 Buford Hwy NE, MS G-23, Atlanta, GA, 30341. Telephone: 770-488-8126; Fax: 770-488-8275; Email: dhowerton@cdc.gov. SummaryUnder the Clinical Laboratory Improvement Amendments of 1988 (CLIA), simple, low-risk tests can be waived and performed with no routine regulatory oversight in physicians' offices and various other locations. Since CLIA was implemented, waived testing has steadily increased in the United States. Surveys conducted during 1999--2004 by the Centers for Medicare & Medicaid Services and studies funded by CDC during 1999--2003 evaluated testing practices in sites holding a CLIA Certificate of Waiver (CW). Although study findings indicate CW sites generally take measures to perform testing correctly, they raise quality concerns about practices that could lead to errors in testing and poor patient outcomes. These issues are probably caused, in part, by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Committee for conducting quality waived testing. These recommendations include considerations before introducing waived testing, such as management responsibility for testing, regulatory requirements, safety, physical and environmental requirements, benefits and costs, staffing, and documentation. They also cover good laboratory practices for the three phases of testing: 1) before testing (test ordering and specimen collection), 2) during testing (control testing, test performance, and result interpretation and recording), and 3) after testing (result reporting, documentation, confirmatory testing, and biohazard waste disposal). They are intended to be used by those who would benefit from improving their knowledge of good laboratory practices. Continued monitoring of waived testing, with a focus on personnel education and training, is needed to improve practices and enhance patient safety as waived testing continues to increase. IntroductionLaboratory testing plays a critical role in health assessment, health care, and ultimately, the public's health. Test results contribute to diagnosis and prognosis of disease, monitoring of treatment and health status, and population screening for disease. Laboratory testing affects persons in every life stage, and almost everyone will experience having one or more laboratory tests conducted during their lifetime. An estimated 7--10 billion laboratory tests are performed each year in the United States (1,2), and laboratory test results influence approximately 70% of medical decisions (2--4). Increasingly, these decisions are based on simple tests performed at the point-of-care using devices that are waived from most federal oversight requirements (and are thus designated as waived tests), including requirements for personnel qualifications and training, quality control (QC) (unless specified as required in the test system instructions), proficiency testing (PT), and routine quality assessment. Advances in technology have made tests simpler, contributing to this shift in testing. In the past, tests such as prothrombin time, cholesterol, and glucose either used complex manual methodologies or were performed using sizable instrumentation suitable for use by highly trained personnel in traditional clinical laboratory settings. Many tests can now be performed using compact or hand held devices by personnel with limited experience and training. These advances have enabled more testing to be performed in emergency departments, hospital rooms, and physicians' offices and in nontraditional testing sites such as community counseling centers, pharmacies, nursing homes, ambulances, and health fairs. Since the 1992 inception of the program implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA), the numbers of waived tests and the sites that perform them have increased dramatically. This trend is expected to continue as laboratory testing technology continues to evolve. The purpose of this report is to highlight quality issues identified in waived testing sites on the basis of surveys conducted on-site by the Centers for Medicare & Medicaid Services (CMS) during 1999--2004 and studies of waived testing practices funded through CDC during 1999--2003. In addition, this report presents recommendations developed by the Clinical Laboratory Improvement Advisory Committee (CLIAC) for improving the quality of waived testing. By following these recommendations, errors that could potentially lead to patient harm and the associated morbidity and mortality can be prevented. BackgroundCLIA Requirements for Waived TestingAll facilities in the United States that perform laboratory testing on human specimens for health assessment or the diagnosis, prevention, or treatment of disease are regulated under CLIA (5). The CLIA program is administered by CMS and is implemented through three federal agencies---CDC, CMS, and the Food and Drug Administration (FDA). When CLIA was implemented in 1992, CLIAC was chartered to provide scientific and technical advice and guidance to the U.S. Department of Health and Human Services (HHS) about laboratory standards and their impact on medical and laboratory practice. The committee consists of 20 members selected by the HHS secretary from authorities knowledgeable in the fields of laboratory medicine, pathology, public health, and clinical practice and includes consumer representatives and an industry liaison. CLIAC also includes three ex officio members from CDC, CMS, and FDA. By law, CLIA regulations are based on a complexity model, with more complicated testing subject to more stringent requirements (6). The three categories of testing for CLIA purposes are waived, moderate complexity (including the provider-performed microscopy procedures [PPMP] subcategory), and high complexity. Facilities performing only waived tests have no routine oversight and no personnel requirements and are only required to obtain a Certificate of Waiver (CW), pay biennial certificate fees, and follow manufacturers' test instructions. Tests can be waived under CLIA if they are determined to be "simple tests with an insignificant risk of an erroneous result" (5). Eight tests were included in the 1992 CLIA regulations (a ninth test was subsequently added) as meeting these criteria and later, the FDA Modernization Act of 1997 clarified that tests cleared by FDA for home use are automatically waived. An additional route to waiver exists through a process in which FDA evaluates studies and other information submitted by manufacturers to demonstrate that a test meets the waiver criteria of being simple and having a low risk for error. Approximately 1,600 test systems representing at least 76 analytes are waived under CLIA (Table 1). Scope of Waived TestingSites performing only waived tests comprise 58% (105,138) of the approximately 180,000 laboratory testing sites in the United States (Table 1, Figure 1). Waived testing performed in these sites is often wellness testing, screening tests, or other critical testing that introduces a large population of persons into the health-care setting. Although the testing performed in CW sites accounts for <10% of the total U.S. testing volume, this percentage has been increasing each year since the CLIA program began (Table 1). Most testing is not waived and is typically performed in hospital or reference laboratories (Certificate of Compliance and Certificate of Accreditation), which comprise 20% of the total number of testing sites (Figure 1). The remaining testing sites (22%) have PPMP certificates, meaning that in addition to waived tests, direct microscopic examinations of certain specimens can be performed as part of the patient's examination by that patient's physician or midlevel health-care practitioner. An increasing shift toward waived testing has resulted in a corresponding increase in health-care expenditures for this testing. Medicare Part B, the federal medical insurance program for persons aged >65 years and certain disabled persons, covers diagnostic laboratory testing. Payment data for 2004, provided by CMS, indicated that of the $3,494,840,086 spent on reimbursed laboratory testing for that year, $128,169,398 (3.7%) was for waived tests. The volume of Medicare Part B reimbursed waived laboratory testing in 2004 represented 8% of the total reimbursed testing volume for that year, a 57% increase over the volume in 2000 (Table 1). Patient Safety Concerns Related to Waived TestingEfforts to reduce medical errors, improve health-care quality, and increase patient safety have been gaining national attention. A report issued in 1999 by the Institute of Medicine (IOM) presented a national agenda to address these issues and recommended strategies for change that included the implementation of safe practices at the health-care delivery level (7). As described in the IOM report, errors most often occur when multiple contributing factors converge, and preventing errors and improving patient safety require a systems approach. Five years after this seminal report, small but consequential changes have occurred that have shifted the focus to improving systems, engaging stakeholders, and motivating health-care providers to adopt new safe practices (8). Although by law waived tests should have insignificant risk for erroneous results, these tests are not completely error-proof and are not always used in settings that employ a systems approach to quality and patient safety. Errors can occur anywhere in the testing process, particularly when the manufacturer's instructions are not followed and when testing personnel are not familiar with all aspects of the test system and how testing is integrated into the facility's workflow. Although data have not been systematically collected on patient outcomes with waived testing, adverse events can occur (9). Some waived tests have potential for serious health impacts if performed incorrectly. For example, results from waived tests can be used to adjust medication dosages, such as prothrombin time testing in patients undergoing anticoagulant therapy and glucose monitoring in diabetics. In addition, erroneous results from diagnostic tests, such as those for human immunodeficiency virus (HIV) antibody, can have unintended consequences. The lack of oversight and requirements for personnel qualifications and training for an increasingly large number of CW sites is a concern and could contribute to errors and patient harm. During 1999--2001, CMS conducted on-site surveys of a representative sample of CW sites in 10 states to assess the quality of testing in these sites. These pilot surveys identified quality issues that could result in medical errors (10). Contributing factors included inadequate training in good laboratory practices and high turnover rates of testing personnel. As a result, during 2002--2004, CMS conducted nationwide on-site surveys of CW facilities to collect additional data that would provide an assessment of testing, promote good laboratory practices and encourage improvement through educational outreach, and make recommendations on the basis of cumulative survey findings. The data collected from these surveys, along with data on waived testing practices gathered through CDC-funded studies conducted during 1999--2003 by the state health departments of Arkansas, New York, and Washington (collectively referred to as the Laboratory Medicine Sentinel Monitoring Network [LMSMN]), support the initial CMS findings of gaps in good laboratory practices in these sites (11--16). In addition, a 2001 report issued by the HHS Office of Inspector General (OIG), following their investigation of CLIA certification and enrollment processes, identified the lack of routine on-site visits to CW sites by surveyors representing state agencies and private sector accreditation organizations as presenting vulnerabilities in these sites. The OIG report indicated that approximately half of the state respondents reported problems related to quality issues with the waived laboratories in their states (e.g., failure to follow manufacturers' instructions or failure to identify incorrect results and performing unauthorized testing) (17). The concerns noted by states were similar to those identified in the CMS pilot studies. CLIAC ResponseAn initial CMS report of its 2002--2003 survey findings, presented to CLIAC in 2004, supported earlier concerns about the quality of testing practices and the need for education and training of testing personnel in CW sites. In response, the committee recommended publication of the 2002--2004 CMS data in conjunction with other data pertinent to waived testing performance along with recommendations for good laboratory practices for waived testing sites. This information would then be available to provide guidance to physicians, nurses, and other health-care providers in CW facilities. As a result, a workgroup was appointed to consider practices associated with the waived testing process and their impact on the quality of waived testing. This workgroup was comprised of key stakeholders in waived testing (i.e., CLIAC members; physicians; nurses; laboratorians; manufacturers; distributors; and representatives from CDC, CMS, and FDA). In its evaluations, the workgroup considered existing practice guidelines from professional organizations, waived testing recommendations from CMS, personal and professional experience, and publications related to waived testing. The workgroup's findings were presented to CLIAC for its deliberations at the February 2005 meeting, at which time CLIAC provided recommendations to HHS concerning good laboratory practices for waived testing sites. CLIAC supported publication of the recommendations, along with the data from the studies of CW sites, and suggested the publication could serve as a comprehensive source document that could be used to develop additional educational tools appropriate for specific target audiences. Surveys of Waived Testing SitesMethodsDuring 2002--2004, approximately 150 CMS and state agency surveyors conducted on-site surveys nationwide using a questionnaire at 4,214 sites performing testing under a CLIA CW. Surveyors self-selected CW sites on the basis of test volume, location, and facility types. Different facilities were surveyed each year so that no repetition exists among CW sites represented in the CMS data in this report. LMSMN obtained additional waived testing data from 1999--2003. Within LMSMN, the Washington State Department of Health established the Pacific Northwest Sentinel Network (PNWSN), which included approximately 650 waived and nonwaived laboratories in Alaska, Idaho, Oregon, and Washington. The Arkansas Sentinel Network (ASN) consisted of 94 local health units integrated into the state health agency (mostly waived testing sites) and approximately 600 waived and nonwaived laboratories in Arkansas and surrounding states. PNWSN and ASN gathered data about waived testing practices through questionnaires mailed to network members (11). The New York Sentinel Network (NYSN) consisted of approximately 600 limited service laboratories (facilities other than physician office laboratories [POLs] that perform only waived tests and PPMP). NYSN collected its data through on-site surveys during which waived testing practices were assessed by surveyor observation and record reviews (11). Survey FindingsDemographics CMS surveyed 4,214 CW sites during April 15, 2002--November 12, 2004. This included 897 sites in 2002, 1,575 sites in 2003, and 1,742 sites in 2004. Of the CW facility types surveyed, POLs compose the largest percentage (47%), followed by skilled nursing facilities (14%) (Table 2). The CW sites surveyed estimated performing a broad range of annual test volumes (Figure 2). Of the facilities surveyed by CMS during 2003--2004 (2002 data not available), 90% reported that they performed no more than five different waived tests, and 99% performed no more than 10 different waived tests. Although the exact volume of each test performed per site is not known, on the basis of the number of sites testing for each analyte, the five most commonly performed waived tests were identified as glucose, dipstick urinalysis, fecal occult blood, urine human chorionic gonadotropin (hCG) (visual color comparison), and group A streptococcal antigen (direct test from throat swabs) (Figure 3). This correlates with data for the top five waived tests identified through the LMSMN, especially for POLs (11). Although not among the most commonly performed, waived tests are available for certain infectious diseases of public health significance and were reportedly performed by CW sites in the CMS surveys (influenza, 46 sites; HIV, four; and Lyme disease, one). Personnel and Training Under CLIA, no education or training is required for the director or testing personnel in CW sites. The educational background and qualifications for directors and testing personnel at CW sites were collected as part of the CMS surveys and by LMSMN (PNWSN and NYSN). The CMS surveys indicated that in 69% of CW sites, physicians served as directors, followed by nurses (17%) (Table 3). Similarly, 59% of the PNWSN CW site directors were physicians, with the remaining 41% having other backgrounds or degrees (12). For CW testing personnel, according to the CMS data, the top four categories were nurses (46%), medical assistants (25%), physicians (9%), and high school graduates (7%) (Table 3). NYSN reported that registered nurses (RNs) and licensed practical nurses (LPNs) served as testing personnel in 84% of the limited service laboratories they surveyed (13). Trained laboratorians (i.e., medical technologists and medical laboratory technicians) accounted for 2% of laboratory directors and testing personnel in the CW sites surveyed by CMS and a smaller percentage in the limited service laboratories surveyed by the NYSN (13). CMS surveys indicated that 43% of CW sites experienced a change in testing personnel during the preceding 12 months. Among the top categories of testing personnel in the PNWSN, turnover rates were highest for medical assistants (17%), followed by LPNs (13%), RNs (9%), and physicians (2%) (14). Although the majority of CW sites in the CMS surveys (90%) reported that new personnel were trained, fewer sites (85%) evaluated staff to ensure competency. Data identifying who provided training were not submitted for all sites in the surveys. However, according to the CW sites that provided this information for 2003--2004 (Table 4), nurses most frequently provided waived test training (33%), followed by the manufacturer or sales representatives (15%). Findings from a PNWSN study indicated that the highest percentage of personnel were initially trained by another employee (25%) or trained themselves by using instructions provided with the waived test system (17%) (15). Another PNWSN study indicated that most training (77%) took place in a day or less (14). Comments from this study reflected the thinking that training is not always necessary or that minimal time should be spent on training because persons have been trained in school or on other jobs. The time spent on training was not captured as part of the CMS surveys. Testing Practices The CMS surveys indicated that the majority of the CW sites were aware of and followed some practices for ensuring the accuracy and reliability of their testing. However, lapses in quality were identified at certain sites, some of which could result in patient harm. In some instances, CW sites were determined to be performing testing that was an imminent and serious threat to the public's health because they were performing nonwaived testing in the absence of CLIA-required quality measures. The CMS surveys indicated that 5% of CW sites were conducting tests that were not waived, the most frequently performed nonwaived procedures (72%) being direct microscopic examinations (e.g., potassium hydroxide preparations, wet mounts, or urine sediment examinations). Surveyed CW testing sites also reported performing various other nonwaived tests (e.g., urine and throat cultures, Rh antigen testing, and the use of glucometers to perform diagnostic glucose tolerance testing [an intended use not specified in manufacturers' instructions]). When performing nonwaived tests, surveyors noted that, in some instances, the sites were not meeting CLIA requirements for qualified personnel, QC, PT, or test system maintenance. In addition, these sites did not have adequate records of their testing activities, including test system procedures, training records, or other documentation. Of the CW facilities CMS surveyed, 12% did not have the most recent instructions for the waived test systems they were using, and 21% of the sites reported they did not routinely check the product insert or instructions for changes to the information (Table 5). On the basis of manufacturer's instructions, 21% of the CW sites did not perform QC testing as specified, and 18% of the sites did not use correct terminology or units of measure when reporting results. Among other quality deficiencies identified were failure to adhere to proper expiration dates for the test system, reagents, or control materials (6%) and failure to adhere to the storage conditions as described in the product insert (3%). Six percent of CW sites did not perform follow-up confirmatory tests as specified in the instructions for certain waived tests (e.g., group A streptococcal antigen), and 5% did not perform function checks or calibration checks to ensure the test system was operating correctly. Findings from the LMSMN studies were similar to the CMS findings for these quality deficiencies (11). Although not usually specified in the product insert (and therefore not a CLIA requirement), proper documentation and recordkeeping of patient and testing information are also important elements of good laboratory practices. CMS surveys indicated that 45% of CW sites did not document the name, lot number, and expiration dates for tests performed; 35% did not maintain logs with records of their QC testing; 31% did not maintain a log or record of tests performed; and 9% did not require a requisition or test orders documented in a patient chart before performing a test (Table 5). NYSN observed similar findings but noted increased compliance with state requirements for documentation/recordkeeping when laboratories had formal affiliations with New York State-licensed laboratories (11). DiscussionThe findings from the CMS surveys and LMSMN studies indicated that the majority of CW testing sites performed testing correctly and provided reliable service. However, in CW sites, most directors and testing personnel did not have formal laboratory training or testing experience, there was a high turnover of personnel, and lapses in following manufacturers' instructions and instituting practices to ensure the quality of the testing were noted. The survey findings indicated that 485 (12%) of the 4,214 CW sites surveyed did not have the current manufacturers' instructions available, and 701 (21%) of the 3,317 sites surveyed during 2003--2004 did not check to be sure there had been no changes to the instructions. Test system instructions can change over time and CW sites sometimes switch test systems that could have different instructions. CMS survey results also indicated that, in varying proportions, when CW sites had the current instructions, they did not follow critical steps in the testing process (e.g., performing QC testing, reporting results correctly, adhering to expiration dates and appropriate storage requirements, and performing test system function checks or calibration checks). This is a concern because the only CLIA requirement for performing waived testing is to follow the manufacturer's instructions. Neglecting to follow instructions could cause inaccurate test results that could lead to incorrect diagnoses, inappropriate or unnecessary medical treatment, and poor patient outcomes. CMS surveys indicated that certain CW sites (5%) were performing testing more complex than waived testing without taking required measures to ensure quality. In certain CW sites, nonwaived microscopic examinations were being performed by personnel who lacked the education and training needed to develop the interpretive and judgment skills necessary to accurately perform these procedures. In addition, measures such as QC, PT, adequate documentation, and monitoring are required to ensure the accuracy and reliability of nonwaived test results. Although direct microscopic examinations can be conducted by a physician or midlevel health-care practitioner as part of a patient examination, testing must be conducted under a CLIA PPMP certificate. The quality issues identified through these surveys might have been caused, in part, by high turnover rates of testing personnel in CW sites, inadequate training with respect to waived testing, and lack of understanding of good laboratory practices, including the importance of following all aspects of the manufacturers' instructions. Although the study results indicated that most testing personnel were trained, they were often trained for minimum periods by persons who did not have formal education or training in clinical laboratory testing and who might not have understood the importance of measures to ensure quality testing. Certain testing personnel also were self-trained. In addition, when testing personnel were not evaluated to determine their competency level following training or on an ongoing basis, no assessment was conducted to determine whether the training was effective. The data demonstrate a need for educational information among CW site directors and testing personnel about the importance of following manufacturers' instructions, adhering to expiration dates, performing QC testing, and proper documentation and recordkeeping. One of the recommendations in the 2001 OIG report was that CMS should provide educational outreach to directors of waived and PPMP laboratories about the CLIA requirements (17). The findings in the 2002--2004 CMS surveys are subject to at least three limitations, and caution should be used in extrapolating the survey data to make generalizations about waived testing. First, the CMS surveys were not intended to be a scientific study of a random sample of CW sites. Waived testing data were collected by CMS to provide an assessment of testing practices, promote good laboratory practices, and encourage improvement through educational outreach. Although surveyors attempted to include a wide variety of CW sites in the sample, the sites were self-selected by surveyors and selection was based, to some degree, on convenience to the surveyors and willingness of the sites to participate in the voluntary surveys. However, few sites refused to participate in the surveys. Overall, the sites represent a nationwide sample and the distribution of CW facility types is similar to the distribution of CW facility types in the United States (Table 2). In addition, the 2002--2004 CMS survey findings resulted in the same general conclusions as the earlier CMS pilot studies, which were conducted on a random sample of laboratories (10). Second, the CMS data were collected and entered into the database by a large number of persons, introducing variability. Although training was provided before the surveys were conducted, the intent of the survey questions was subject to individual interpretation. Because the phrasing of some questions differed slightly from 2002 to 2003--2004, in certain cases, the meanings of the questions also changed. Finally, the CMS surveys did not assess the frequency of erroneous test results in CW sites or whether lapses in following manufacturers' instructions directly affected test results or patient outcomes. Similar limitations to these were identified in the LMSMN studies (11). The findings of the CMS and LMSMN studies are strikingly similar. Even though the majority of CW sites meet the CLIA requirement to follow manufacturers' instructions for test performance, and many sites follow additional good laboratory practices, over the years these studies have demonstrated that a persistent percentage of CW sites do not meet minimal requirements and are not aware of recommended practices to help ensure quality testing. Because surveying all CW sites is not feasible, the proposed actions to improve and promote quality testing in CW sites emphasize the importance of education and training for CW site directors and testing personnel. To provide a guide that can be adapted for use, either in part or as a whole, by persons or facilities considering the initiation of waived testing and personnel performing waived testing, CLIAC provided recommendations for good laboratory practices. By implementing these recommendations, CW sites could improve quality, reduce testing errors, and enhance patient safety. Recommended Good Laboratory PracticesOverviewThese recommendations are intended to promote the use of good laboratory practices by physicians, nurses, and other providers of waived testing in a variety of CW sites. They were developed on the basis of recommendations and other resources that provided additional information for promoting patient safety and the quality of CLIAC waived testing in laboratories or nontraditional testing sites (18--22). These recommendations address decisions that need to be made and steps to be taken as a facility begins offering waived testing or adds a new waived test. They also address developing procedures and training CW personnel and describe recommended practices for each phase of the total testing process, or path of workflow, including the important steps or activities before, during, and after testing. The activities that occur in each of these phases are critical to providing quality testing (Table 6). Considerations Before Introducing Waived Testing or Offering a New Waived TestForethought, planning, and preparation are critical to initiating high-quality waived testing in any type of setting. This section describes factors to consider before opening a waived testing site or offering an additional waived test. Questions to address include the following:
Management Responsibility Each testing site should identify at least one person responsible for testing oversight and decision-making, later referred to as the CW site director. In POLs, this might be a physician or someone in a senior management position who has the appropriate background and knowledge to make decisions about laboratory testing. Ideally, the person signing the CW application (CMS Form 116) is responsible for management of the testing operations. The management staff should demonstrate a commitment to the quality of testing service by complying with applicable regulatory requirements and promoting good laboratory practices. Regulatory Requirements CLIA certification. Each site offering only waived testing that is not included under any other type of CLIA certificate must obtain a CLIA CW before testing patient specimens. Certain sites offering waived testing can be certified as part of a larger health-care organization that holds a CLIA Certificate of Compliance or Certificate of Accreditation. In addition, certain public health testing sites offering only waived testing can be included under a limited public health or mobile testing exception. A valid CLIA certificate is required for Medicare reimbursement. To apply for a CLIA certificate, CMS Form 116 (http://www.cms.hhs.gov/clia/cliaapp.asp) must be completed and sent to the state agency for the state in which the testing site is located. This form asks for specific information, including the type of testing site (laboratory type), hours of operation, estimated total annual volume of waived testing, and the total number of persons involved in performing waived testing. The form must be signed by the facility owner or the facility director. Specific state agencies and contacts are available at http://www.cms.hhs.gov/clia/ssa-map.asp. The state agency will process the application and send an invoice for the registration fee. If additional assistance is required, contact the appropriate CMS regional office (http://www.cms.hhs.gov/clia/ro-map.asp). CLIA requirements that apply to testing sites operating under a CW include the following:
State and local regulations. States and local jurisdictions vary as to the extent to which they regulate laboratory testing. Some states and localities have specific regulations for testing, some require licensure of personnel who perform testing, and some have phlebotomy requirements. State and local jurisdictions often regulate biohazard safety, including handling and disposal of medical waste. The person responsible for testing oversight should ensure that all state and local requirements are met. These requirements might be more or less stringent than federal requirements. When state or local regulations governing laboratory testing are more stringent than the federal CLIA requirements, they supersede what is required under CLIA. Safety requirements. The Occupational Safety and Health Administration (OSHA) and individual state standards require employers to provide a safe and healthy work environment for employees. Each CW site must comply with OSHA standards pertinent to workplace hazards (23). Regulatory requirements for all OSHA standards, including specific information for medical and dental offices (24), are available at http://www.osha.gov and by telephone, 800-321-6742. The OSHA Bloodborne Pathogens Standard applies to sites where workers have potential occupational exposure to blood and infectious materials (25). The requirements for compliance with this standard include, but are not limited to:
Specific information on the Bloodborne Pathogens Standard and needlestick prevention is available at http://www.osha.gov/SLTC/bloodbornepathogens/index.html. CDC and the Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) have also published information about biosafety and precautions for preventing transmission of bloodborne pathogens in the workplace (26--30). Privacy and confidentiality requirements. The Health Insurance Portability and Accountability Act of 1996 (HIPAA) established federal privacy standards to protect patients' medical records and other health information provided to health plans, doctors, hospitals, and other health-care providers. Under HIPAA, CW sites are required to establish policies and procedures to protect the confidentiality of health information about their patients, including patient identification, test results, and all records of testing. These medical records and other individually identifiable health information must be protected, whether on paper, in computers, or communicated orally. In addition, CW sites should be aware that applicable state laws that provide more stringent privacy protections for patients supersede HIPAA. Additional information on HIPAA is available at http://www.hhs.gov/ocr/hipaa. Physical Requirements for Testing Testing should be performed in a separate designated area where adequate space to safely conduct testing and maintain patient privacy is available. In addition, some tests have specific environmental requirements described in the manufacturer's product insert that need to be met to ensure reliable test results. Meeting these environmental conditions can be challenging in nontraditional settings (e.g., health fairs) or community outreach venues (e.g., shopping malls, meeting rooms, parks, parking lots, mobile vans, and buses). Factors to consider include:
Benefit and Cost Considerations Evaluating the benefits of a particular test. Evaluate the test system, its intended use, performance characteristics, and the population to be tested when assessing whether to introduce waived testing or a new waived test. Information for this evaluation can be obtained from the test manufacturer's product insert (Table 7) or by speaking with the manufacturer's technical representatives. Specific considerations include:
Cost considerations. A fiscal assessment of testing is part of a good management program. Before offering a new test, consider the level of reimbursement and factors that contribute to total test cost. These factors include:
Personnel Considerations Personnel competency and turnover are important factors affecting the quality and reliability of waived testing results. No CLIA requirements exist for waived testing personnel qualifications; however, applicable state or local personnel regulations must be met. Personnel issues to consider include:
After the decision is made to offer waived testing, it is good practice to develop written policies and procedures so that responsibilities and testing instructions are clearly described for the testing personnel and facility director. The testing procedures form the basis of training for testing personnel. These procedures should be derived from the manufacturer's instructions and should be in a language understandable to testing personnel. Written Test Procedures To comply with CLIA requirements and provide accurate testing, CW sites must adhere to the manufacturer's current testing instructions. These instructions, as outlined in the product insert, include directions for specimen collection and handling, control procedures, test and reagent preparation, and instructions for test performance, interpretation, and reporting (Table 7). In addition, certain manufacturers provide quick reference instructions formatted as cards or small signs containing essential steps in conducting a test. Quick reference instructions should be clearly posted where testing is performed. The specific test system name should be on the quick reference instructions to avoid confusion. A comprehensive procedure manual is a valuable resource for CW sites. Although product inserts can be used as test procedures, these instructions will typically need to be supplemented with testing information that is unique to the CW site's operations and workflow (31). A procedure manual can also include examples of forms used (e.g., charts to record daily test kit storage temperatures, infectious disease reporting forms, or logs for recording control testing and test results) and check lists for personnel training. New testing procedures should be reviewed and signed by the CW site director before incorporating them into the procedure manual. The manual should be updated as tests or other aspects of the testing service change and should be reviewed by the director whenever changes are made. When procedures are no longer used, they should be removed from the manual and retained with a notation of the dates during which they were in service. When writing procedures for each CW site, it might be helpful to:
Personnel Training Trained and competent testing personnel are essential to good quality testing and patient care. Data from CDC and CMS surveys demonstrate that waived testing sites are subject to a high rate of personnel turnover. Personnel should be trained and competent in each test they will perform before reporting patient results (32,33). In addition, training should include aspects of safety (including Universal Precautions) and QC. The CW site director or other person responsible for overseeing testing should ensure that testing personnel receive adequate training and are competent to perform the procedures for which they are responsible. Training checklists are helpful to ensure the training process is comprehensive and documented. The training process. Training should be provided by a qualified person (e.g., experienced co-worker, facility expert, or outside consultant) with knowledge of the test performance, good laboratory practices, and the ability to evaluate the efficacy of the training. On-the-job training should include the following steps:
Training resources. Resources for training are available from various sources. Tools for training continue to evolve and are not limited to traditional methods. Instructional videos, workshops, computer-based programs, and other methods can be used. The manufacturer's test system instructions and instrument operating manuals should be the primary resource for information and training in CW sites. Other sources for training on waived testing or specific tests include:
Competency Assessment To ensure testing procedures are performed consistently and accurately, periodic evaluation of competency is recommended, with retraining, as needed, on the basis of results of the competency assessment (32). Assessment activities should be conducted in a positive manner with an emphasis on education and promoting good testing practices. Competency can be evaluated by methods such as observation, evaluating adequacy of documentation, or the introduction of mock specimens by testing control materials or previously tested patient specimens. External quality assessment or evaluation programs, such as voluntary PT programs, are another resource for assessment. Additional Measures to Help Testing Staff Ensure Reliable Results The CW site director or person overseeing testing should promote quality testing and encourage staff to ask questions and seek help when they have concerns. Recommendations include:
Preparations before performing patient testing are a critical element in producing quality results. Paying attention to test orders, properly identifying and preparing the patient, collecting a good quality specimen, and setting up the test system and testing area all contribute to reliable test results. Test Orders, Patient Identification, and Preparation Before collecting the specimen, confirm the test(s) ordered and the patient's identification and verify that pretest instructions or information, as applicable, have been provided. This includes:
Specimen Collection and Handling The product insert provides details on proper collection, handling, and storage of patient specimens. Collect waived test specimens exactly as described in the test system instructions, using the appropriate collection device and method to obtain a quality specimen (33--36). Improperly collected, stored, or compromised specimens should not be tested. Specimens and, in some cases, test devices need to be appropriately labeled to prevent mix-up. Waived test specimens. Waived tests are approved for use only with direct, unprocessed specimens that do not require operator manipulation (Table 8). Specimens that are processed or manipulated by the user (e.g., serum or plasma) require centrifugation, dilution, extraction, or other preparation steps that require special training or instrumentation and are not appropriate for waived tests. Sometimes, tests can be performed using both processed and unprocessed specimen types, but are waived only for the unprocessed specimens, in which case the product insert should identify the appropriate specimen for the waived test. For example, a single product insert might include instructions for performing a waived test using unprocessed whole blood and for performing the same test using plasma, which would not be waived. Other examples include group A streptococcal antigen testing, which is waived only when performed on a throat swab and not when performed on a microbiology culture, and visual color comparison tests for hCG (pregnancy tests) using urine that are waived, whereas serum or plasma hCG tests are not waived. Specimen collection. The person collecting the patient specimen or giving the collection instructions should have a thorough understanding of the specimen type, proper collection method (including the need to wear gloves or other PPE as appropriate), and handling to assure a quality specimen (33--36). Directions for specimen collection, handling, and storage are included in the product insert and must be followed explicitly. For example, instructions might specify one drop of capillary blood or include precautions to use the second drop of blood from a fingerstick rather than the first. When gloves are worn during specimen collection, they should be removed and discarded in an appropriate waste receptacle before contact with another patient. Hand hygiene should be performed between patients. Collection devices. Manufacturers might provide or specify specimen collection devices. These devices, whether supplied with the test system or specified in the product insert, are integral to the test system and should be used to ensure the correct specimen type and volume to provide reliable results. Containers and collection devices might have additives that affect the specimen or are part of the test and should not be substituted or altered. For example, throat swab collection kits used with group A streptococcal antigen tests might look the same; however, they might be made from a variety of fibers or contain different materials that could interfere with the test or affect organism viability. Whole blood capillary tubes (e.g., used for cholesterol, hemoglobin A1C, or Helicobacter pylori testing) can have additives or hold different specimen volumes which affect test reactions and results. Fingerstick and venipuncture collection devices are for one-time use only. Never reuse needles, syringes, or lancets. To avoid transmission of hepatitis B virus, hepatitis C virus, HIV, and other bloodborne pathogens, appropriately discard sharps, lancets, and platforms for spring-loaded lancets and disinfect instruments contaminated by blood (9, 28). Specimen labeling. Labeling procedures should meet the needs of the testing site and should be adequate to prevent specimen mix-up. To prevent errors, always label specimens with pertinent information (e.g., unique patient name or other unique identifier). Depending on workflow, specimen labeling also might include the date and time of collection and identification of the collector. For waived tests in which the specimen is applied directly to the test device (e.g., throat swabs for group A streptococcal antigen), the test strip, cassette, or other device should be labeled with the patient identification before collecting the specimen, especially if more than one test is being performed at the same time. Preparing the Testing Area, Test Materials, and Equipment Preparing the testing area and materials (e.g., kits, reagents, control materials, and equipment) before testing patient specimens is essential to maintaining efficient workflow and good quality testing (Table 9). Before beginning the test, read and understand the test instructions specified in the product insert and included in the CW site's procedures. Verify that the instructions are current for the test in use and that no changes have been made. Do not use product inserts that are out of date for the test system currently in use. When opening a new kit, check for notifications in the external labeling or special notices that might be included with product inserts or packaging. Additional considerations for good testing practices are:
When the testing area is prepared and the specimen has been collected, the process continues to the testing phase. Important activities during this phase include QC testing, test performance, result interpretation and recording. Quality Control Testing Performing QC testing procedures provides assurance that the test performs as expected and alerts the user when problems occur. QC testing is designed to detect problems that might arise because of operator error, reagent or test kit deterioration, instrument malfunction, or improper environmental conditions. Test procedures should describe the type of controls to be used, how to perform QC testing (including QC testing frequency), and actions to be taken when QC results are unacceptable. Types of controls. Two types of controls typically found in waived tests are:
Frequency of control testing. For certain test systems, the product insert describes the minimum conditions or recommended frequencies for testing internal and/or external controls. Each site should determine the appropriate control testing frequency for each test system and the frequency should not be less than specified in the product insert. When determining the frequency for running external controls, consider the robustness of the test, stability of the environment, and skills and knowledge of the testing personnel. At a minimum, external controls should be tested with each new shipment of utilized test devices, when testing a new lot number, and by each new operator before conducting testing. Controls should be tested either before or concurrent with patient specimens by the same personnel who routinely perform patient testing. Corrective action when control testing fails. If controls do not perform as expected, patient testing should not be performed or results reported until the problem is identified and corrected. The product insert should provide information on procedures for handling unexpected control results, identifying sources of error (including interfering substances), and manufacturer contact information for technical assistance. This information might be incorporated into the facility's procedures or posted for quick reference. The test site should have telephone numbers or other contact information readily available (e.g., numbers for manufacturers' technical assistance, the facility's director, consultant, or public health departments). Documentation. Documenting and monitoring control testing results provides an indication that the test was properly performed by the operator and the test system (reagents, instruments, or any components) performed as expected. Records of control results should be periodically reviewed to detect shifts or changes in performance over time. Performing the Test The following points are important to remember when performing the test:
Test Results Interpretation When the test is complete, interpret the results according to instructions in the product insert (including the quick reference guide). Test results are of the following two types:
Resolving Problems If a discrepancy is identified between the patient's test results and the clinical information or if the results are invalid or otherwise compromised, testing should be repeated. Results should not be reported until the problem is resolved. Follow the steps in the product insert to resolve problems with test results. Unitized test system instructions usually suggest repeating the test with a new device and referring to QC or trouble-shooting procedures. If repeat testing does not resolve the problem, contact the manufacturer or product technical representative. Quantitative results can be obtained that are beyond the measuring range of the instrument or test device. Each site should have documentation of quantitative test measuring ranges and a procedure for handling test results that are beyond the reportable ranges, either low or high. Recording Results Record test results according to the site's policy. Results can be recorded directly in a patient's chart, in log books, or on a separate report form. Records or logs of test results should have enough detail so the test site can retrieve information. Quantitative results should be recorded using the units of measurement of the test system. Qualitative test results should be recorded using interpretive words or abbreviations such as positive, negative, reactive or R, or nonreactive or NR instead of symbols like plus and minus (+, -) to help avoid clerical errors because a negative (-) sign can easily be changed to a positive (+) sign. If a test result is not acceptable or requires repeat testing (e.g., out of range or invalid), record the initial result, noting it was unacceptable, take steps necessary to resolve the problem, then record the correct result. Good laboratory practices include recording what happens, whether acceptable or not, and what is done to correct problems encountered during testing. Recommended Practices After TestingAfter-testing activities include issuing test reports, supplemental or confirmatory testing, public health disease reporting (if required), testing area cleanup, biohazard waste disposal, and documentation of testing activities. Test Reports After the completion of the test, results are documented and reported. Patient reports should be legible and reported in a timely manner to the appropriate person. Reports should meet the needs of the testing site and should be appropriately standardized so reports generated on-site are easily distinguishable from referral laboratory reports. Verbal reports of test results should be documented and followed by a written report. Waived testing sites, such as point-of-care sites or physicians' offices, might accurately and legibly record results directly in the patient's record as a matter of practice. If results are not recorded directly in a patient's chart, they should be recorded in a written report format that includes all information needed to correctly identify and interpret the results as determined by the testing site (Table 10). Critical values are test results necessary for patient evaluation or treatment that require immediate notification to the clinician. Each site should define the critical values, if appropriate, for the tests in use and ensure that testing personnel are aware of these values and the procedure for alerting the clinician. Procedures should be in place to ensure documentation of critical values and timely notification of the proper medical personnel. Supplemental or Confirmatory Testing The product insert should explain when supplemental testing is needed to confirm a waived test result or when the test is to be used as part of a multitest algorithm. A confirmatory test could be a different waived test (performed at the testing site or another CW site) or a nonwaived test performed by a CLIA-certified referral laboratory (37) (Table 11). When nonwaived confirmatory testing is needed, the patient can be sent to another facility for specimen collection and testing, or the specimen can be collected at the CW site and sent to a referral laboratory. The CW site should have written policies to ensure confirmatory and supplemental testing is performed when needed. For each waived test that requires additional testing, the CW site should document the processes and procedures necessary to manage referral or confirmatory testing. When a CW site collects specimens for referral, procedures should include the following:
Maintaining records of referred testing is important for patient care and follow-up. Logs and other records should have sufficient information to track and retrieve the test results and reports, such as:
Public Health Reporting Federal and state public health agencies require testing facilities to report confirmed positive results for certain infectious diseases (e.g., HIV, influenza, and Lyme disease) (38,39). Testing facilities should confer with local public health agencies for the most current information on required reporting procedures since diseases identified for reporting can change over time, and state requirements might vary. Biohazard Waste Disposal Dispose of the biohazardous waste generated in specimen collection and testing according to site procedures that need to be in compliance with local ordinances, state, and federal OSHA regulations as previously discussed. Documents and RecordsDocumentation is essential to assure quality waived testing. Proper documentation is necessary for monitoring and assessing test performance, identifying and resolving problems that could affect patient testing, retrieving and verifying information, and maintaining adequate patient and personnel records. Log books or electronic systems can be used for maintaining and tracking information. In some cases, records might be part of the patient's medical chart. Testing records should be maintained in chronological order to facilitate retrieval of information if needed. In addition, control records should be kept in the order in which they were completed so they can easily be compared with test records if there are questions about testing performed within a specific time period. The person responsible for testing oversight and decision-making should review records periodically. State regulations or other governmental agencies might require CW sites to retain documents and records for a specific length of time. Aspects of testing for which records or documentation are recommended include:
Good laboratory practices can be expanded to include assessment activities to evaluate and improve the quality of CW site testing. Assessment activities can be either internal or external, depending on the needs, resources, and practices of the site. Internal Assessment Objective internal assessment offers flexible, low-cost options for evaluating quality such as self-conducted inspections, supervisory review of documented problems that occur in the different phases of the testing process, review of QC documentation, and testing and reporting procedures. Test performance can be assessed, if specimens are suitable, by exchanging specimens with another testing facility using the same test method(s) and comparing the results. Results from these assessment activities should be documented and evaluated, noting any irregularities and the actions taken to resolve problems or improve processes or procedures. External Assessment Because CW sites are not routinely inspected by CMS, voluntary inspections by peers or consultants can offer additional educational opportunities and feedback on current practices along with ideas for quality improvement. Voluntary external inspections evaluate the testing site practices and documentation systems, and a more narrowly focused assessment of test performance can be accomplished by participating in performance evaluation programs or subscribing to PT programs. These programs provide challenge samples to test as if they were patient specimens and the results are evaluated with respect to how close they are to the intended target values. Participation in these types of programs can be used to evaluate overall testing performance and as a training or educational tool for testing personnel. ConclusionThis report summarizes the findings of multiple surveys of sites performing waived testing throughout the United States. Although the surveys were conducted through several mechanisms, the findings lead to similar conclusions about lapses in quality in CW sites, and they highlight the need for additional education and training related to waived testing for CW site directors and testing personnel. The recommendations provided in this report are intended to serve as a guide to improve the quality of testing in CW sites and enhance patient safety. They can be disseminated by a variety of individuals and organizations and adapted for use in different settings where waived testing is conducted. Continued surveillance and monitoring of waived testing performance is needed to determine the effectiveness of these recommendations on protecting and improving the public's health. Acknowledgments The preparers acknowledge the contributions and assistance provided by John Hancock, James Handsfield, MPH, and Rhonda Whalen, MS, of the Division of Public Health Partnerships, National Center for Health Marketing, Coordinating Center for Health Information and Service, CDC; Daralyn Hassan, MS, and the CMS Waived Laboratory Project Team, Division of Laboratory Services, Survey and Certification Group, Center for Medicare and State Operations, CMS. References
Terms and Abbreviations Used in This Report
Clinical Laboratory Improvement Advisory Committee Workgroup Chair: Jared N. Schwartz, MD, Department of Pathology and Laboratory Medicine, Presbyterian Healthcare, Charlotte, North Carolina. Co-Chair: Kathryn M. Foucar, MD, Department of Pathology, University of New Mexico, Albuquerque, New Mexico. Members: Jennifer M. Alfisi, JD, Health Industry Distributors Association, Alexandria, Virginia; Kimberle C. Chapin, MD, Department of Pathology, Rhode Island Hospital, Providence, Rhode Island; Mary Beth Clark, Emory Healthcare, Atlanta, Georgia; Martha H. Crenshaw, MD, Stone Mountain, Georgia; Jacinto Del Mazo, MD, Del Mazo Medical Services, Atlanta, Georgia; Paula W. Garrott, EdM, Clinical Laboratory Science Department, University of Illinois at Springfield, Illinois; Barbara M. Goldsmith, PhD, Caritas St. Elizabeth's Medical Center, Boston, Massachusetts; Luann Ochs, MS, Roche Diagnostics Corporation, Indianapolis, Indiana; Barbara E. Robinson-Dunn, PhD, William Beaumont Hospital, Royal Oak, Michigan; Lou F. Turner, DrPH, North Carolina State Laboratory of Public Health, Raleigh; Robin Weiner, Biosite Inc., San Diego, California; Thomas L.Williams, MD, Methodist Pathology Center, Nebraska Methodist Hospital, Omaha.
Clinical Laboratory Improvement Advisory Committee Chair: SUNDWALL, David N. Sundwall, MD, Utah State Health Department, Salt Lake City, Utah. Executive Secretary: Robert Martin, DrPH, National Center for Health Marketing, CDC, Atlanta, Georgia. Members: Kimberle C. Chapin, MD, Department of Pathology, Rhode Island Hospital, Providence, Rhode Island; Joeline D. Davidson, MBA, West Georgia Health System, LaGrange, Georgia; Kathryn M. Foucar, MD, Department of Pathology, University of New Mexico, Albuquerque; Paula W. Garrott, EdM, Clinical Laboratory Science Dept, University of Illinois at Springfield; Patrick A. Keenan, MD, Department Family Medicine and Community Health, University of Minnesota, Minneapolis; Michael Laposata, MD, Massachusetts General Hospital, Boston; Margaret Mary McGovern, MD, Molecular Genetics Laboratory, Mount Sinai School of Medicine, Mount Sinai Medical Center, New York, New York; Dina R. Mody, MD, The Methodist Hospital, Houston, Texas; Valerie L. Ng, MD, Alameda County Medical Center/Highland Hospital Clinical Laboratory, Oakland, California; Peter J. Gomatos, MD, Fort Lauderdale, Florida; Cyril Michael Hetsko, MD, Madison, Wisconsin; Anthony N. Hui, MD, Northwest Arkansas Pathology Associates, Fayetteville, Arkansas; Kevin P. Kandalaft, Provider Contracting & Provider Services Lovelace Health Systems, Inc., Albuquerque, New Mexico; Barbara E. Robinson-Dunn, PhD, William Beaumont Hospital, Royal Oak, Michigan; Jared N. Schwartz, MD, Department of Pathology and Laboratory Medicine, Presbyterian Healthcare, Charlotte, North Carolina; Albert H. Stahmer, Golden, Colorado; Lou F. Turner, DrPH, North Carolina State Laboratory of Public Health, Raleigh; Thomas L. Williams, MD, Methodist Pathology Center, Nebraska Methodist Hospital, Omaha; Jean Amos Wilson, PhD, Focus Diagnostics, Inc., Cypress, California. Ex Officio Representatives: Steven I. Gutman, MD, Office of In Vitro Diagnostic Device Evaluation & Safety, Food and Drug Administration, Washington, DC; Thomas L. Hearn, MD, National Center for Health Marketing, CDC, Atlanta, Georgia; Judith Yost, MA, Division Laboratories Services, Center for Medicaid and State Operations, Centers for Medicare & Medicaid Services. Liaison Representative: Luann Ochs, MS, Roche Diagnostics Corporation, Indianapolis, Indiana. Table 1 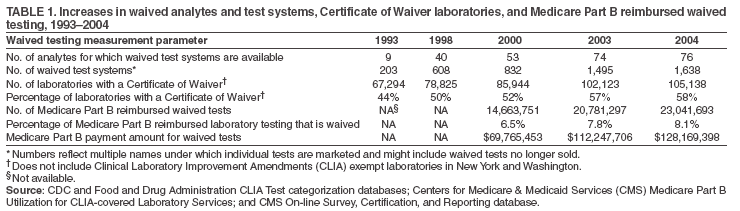 Return to top. Figure 1 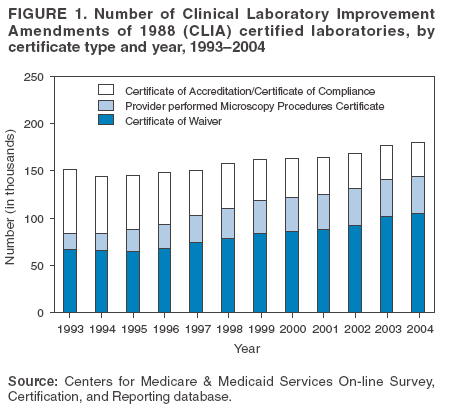 Return to top. Table 2 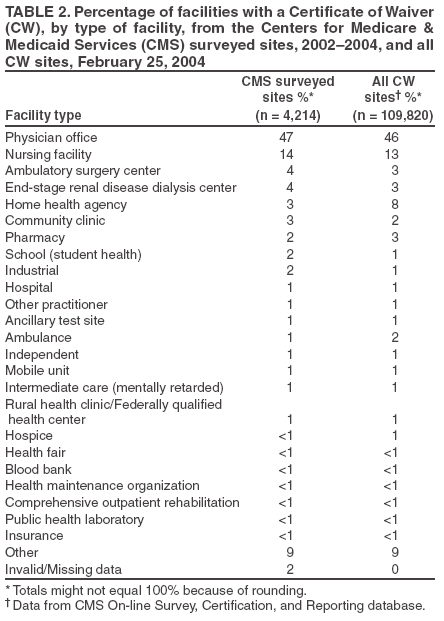 Return to top. Figure 2 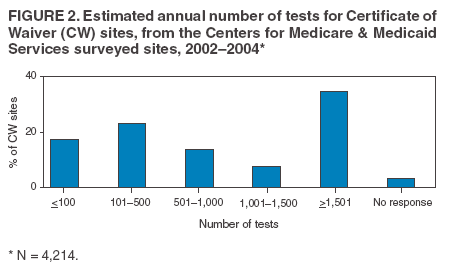 Return to top. Table 3  Return to top. Figure 3 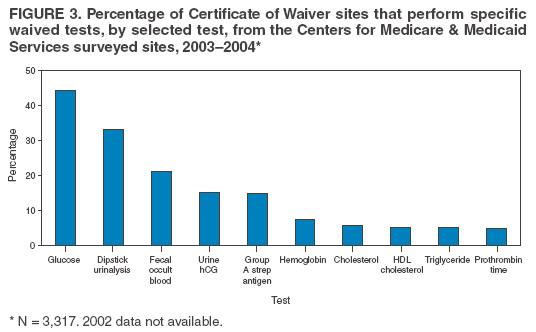 Return to top. Table 4 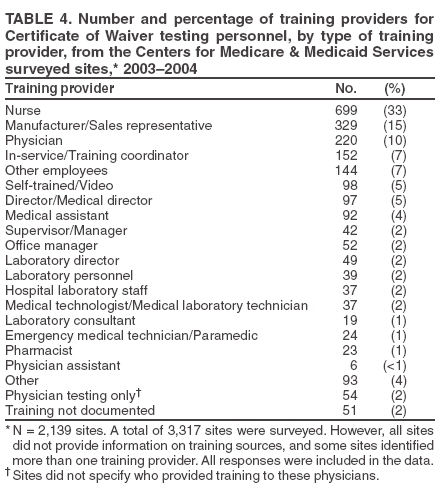 Return to top. Table 5 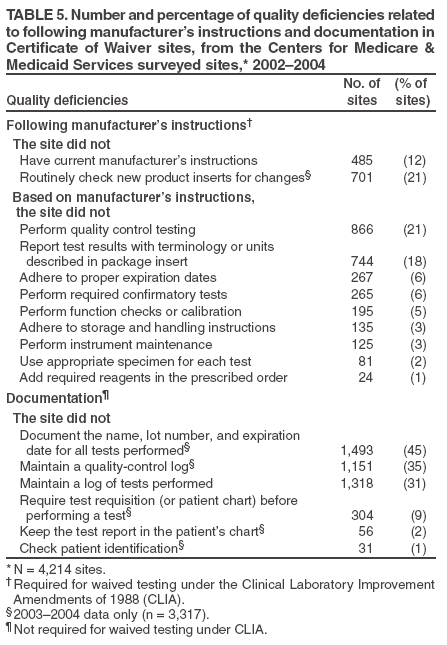 Return to top. Table 6  Return to top. Table 7 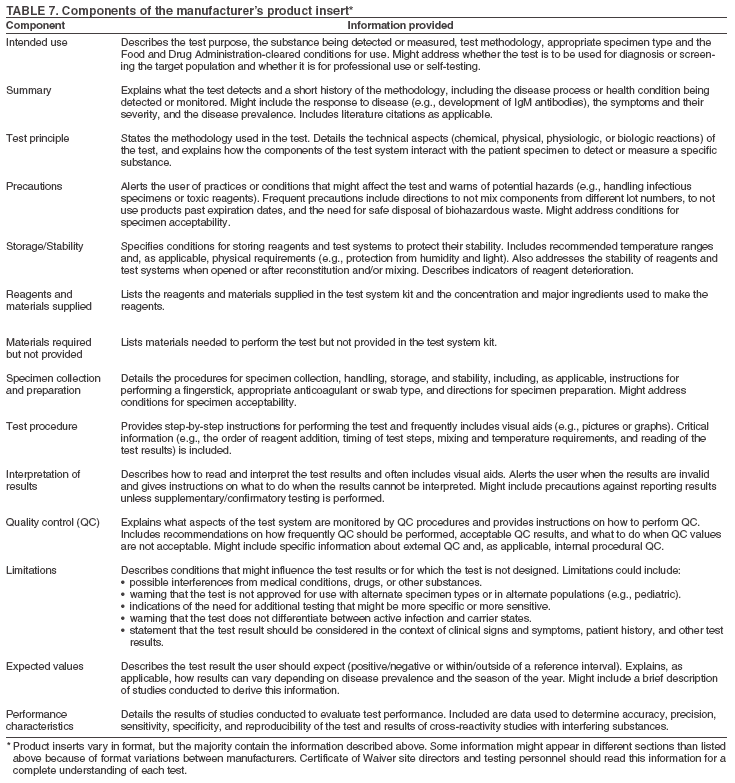 Return to top. Table 8 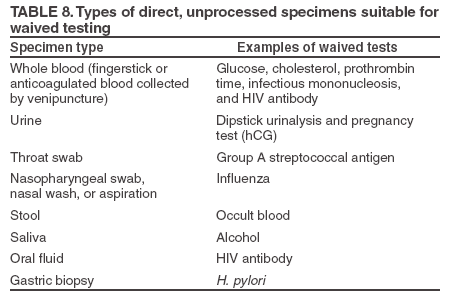 Return to top. Table 9  Return to top. Table 10 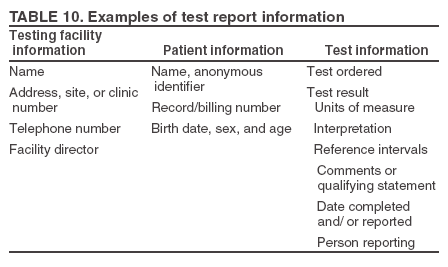 Return to top. Table 11 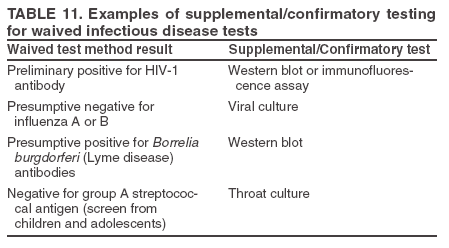 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/26/2005 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|