 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Preventing Tetanus, Diphtheria, and Pertussis Among Adolescents: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis VaccinesRecommendations of the Advisory Committee on Immunization Practices (ACIP)Prepared by
The material in this report originated in the National Immunization Program, Anne Schuchat, MD, Director, Epidemiology and Surveillance Division, Alison Mawle, PhD, Acting Director; and the Office of the Chief Science Officer, Dixie E. Snider, MD, Chief Science Officer, Immunization Safety Office, Frank DeStefano, MD, Acting Director. Corresponding preparer: Karen R. Broder, MD, National Immunization Program, CDC, 1600 Clifton Road, NE, MS E-61, Atlanta, GA 30333. Telephone: 404-639-8538; Fax: 404-639-2483; E-mail: kbroder@cdc.gov. SummaryDuring spring 2005, two tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) products formulated for use in adolescents (and, for one product, use in adults) were licensed in the United States (BOOSTRIX®, GlaxoSmithKline Biologicals, Rixensart, Belgium [licensed May 3, 2005, for use in persons aged 10--18 years], and ADACEL™, sanofi pasteur, Toronto, Ontario, Canada [licensed June 10, 2005, for use in persons aged 11--64 years]). Prelicensure studies demonstrated safety and efficacy against tetanus, diphtheria, and pertussis when Tdap was administered as a single booster dose to adolescents. To reduce pertussis morbidity in adolescents and maintain the standard of care for tetanus and diphtheria protection, the Advisory Committee on Immunization Practices (ACIP) recommends that: 1) adolescents aged 11--18 years should receive a single dose of Tdap instead of tetanus and diphtheria toxoids vaccine (Td) for booster immunization against tetanus, diphtheria, and pertussis if they have completed the recommended childhood diphtheria and tetanus toxoids and whole cell pertussis vaccine (DTP)/ diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) vaccination series (five doses of pediatric DTP/DTaP before the seventh birthday; if the fourth dose was administered on or after the fourth birthday, the fifth dose is not needed) and have not received Td or Tdap. The preferred age for Tdap vaccination is 11--12 years; 2) adolescents aged 11--18 years who received Td, but not Tdap, are encouraged to receive a single dose of Tdap to provide protection against pertussis if they have completed the recommended childhood DTP/DTaP vaccination series. An interval of at least 5 years between Td and Tdap is encouraged to reduce the risk for local and systemic reactions after Tdap vaccination. However, an interval less than 5 years between Td and Tdap can be used; and 3) vaccine providers should administer Tdap and tetravalent meningococcal conjugate vaccine (Menactra®, sanofi pasteur, Swiftwater, Pennsylvania) to adolescents aged 11--18 years during the same visit if both vaccines are indicated and available. This statement 1) reviews tetanus, diphtheria and pertussis vaccination policy in the United States, with emphasis on adolescents; 2) describes the clinical features and epidemiology of pertussis among adolescents; 3) summarizes the immunogenicity, efficacy, and safety data of the two Tdap vaccines licensed for use among adolescents; and 4) presents recommendations for tetanus, diphtheria, and pertussis vaccination among adolescents aged 11--18 years. IntroductionPertussis, an acute, infectious cough illness, remains endemic in the United States despite routine childhood pertussis vaccination for more than half a century and high coverage levels in children for more than a decade (1--4). A primary reason for the continued circulation of Bordetella pertussis is that immunity to pertussis wanes approximately 5--10 years after completion of childhood pertussis vaccination, leaving adolescents and adults susceptible to pertussis (5--10). Among the diseases for which universal childhood vaccination has been recommended, pertussis is the least well-controlled reportable bacterial vaccine-preventable disease in the United States (11,12). In the United States during 1934--1943, an annual average of 200,752 pertussis cases and 4,034 pertussis-related deaths were reported (13). After the introduction of childhood pertussis vaccination during the 1940s, the number of reported pertussis cases declined dramatically, reaching an historic low of 1,010 in 1976 (Figure 1) (1). Since the 1980s, the number of reported pertussis cases has been steadily increasing, especially among adolescents and adults (4,14,15). Possible reasons for the increase in reported pertussis cases include a true increase in the burden of disease and an increase in the detection and reporting of cases; the relative contribution of each of these factors to the increase observed is unclear (4,14--17). Childhood Pertussis Vaccination Policy in the United StatesWhole cell pertussis vaccines became available during the 1920s (18), but pediatric diphtheria and tetanus toxoids and whole cell pertussis vaccine (DTP) was not routinely recommended for children until the 1940s and 1950s (19,20). In 1991, less reactogenic pediatric acellular pertussis vaccine (diphtheria and tetanus toxoids and acellular pertussis vaccine [DTaP]) was first licensed for use in children for the fourth and fifth doses of the 5-dose childhood vaccination series in the United States (21,22), and in 1996, pediatric DTaP was licensed for the first three infant doses (1). In 1997, the Advisory Committee on Immunization Practices (ACIP) recommended that pediatric DTaP be used routinely instead of pediatric DTP as a 5-dose DTaP schedule at ages 2, 4, 6, 15--18 months and 4--6 years (1,23); pediatric DTP has not been available in the United States since 2002 (CDC, unpublished data, 2005). Childhood and Adolescent Tetanus and Diphtheria Vaccination Policy in the United StatesVaccination against tetanus and diphtheria has markedly reduced the number of cases and deaths from tetanus and diphtheria in the United States in all age groups (24). From 1997 through spring 2005, three vaccine formulations against tetanus and diphtheria were recommended for use in the United States: pediatric DTaP routinely for children aged <7 years, pediatric diphtheria and tetanus toxoids vaccine (DT) for children aged <7 years with contraindications or precautions for pertussis components, and adult tetanus and diphtheria toxoids vaccine (Td) routinely for persons aged >7 years (1,24) (Appendix A). The formulation of choice for vaccination of persons aged >7 years has been Td rather than pediatric DT because the lower diphtheria toxoid antigen content of Td induces an adequate immune response and lower rates of adverse reactions in adults than pediatric DT (24--28). To provide continued protection against tetanus and diphtheria, ACIP recommended a booster dose of Td for adolescents (24). Before 1995, the adolescent Td booster was recommended at age 14--16 years, approximately 10 years after completion of the childhood DTP series. In 1995, the first harmonized childhood vaccination schedule endorsed by ACIP, the American Academy of Pediatrics, and the American Academy of Family Physicians recommended lowering the age for Td administration to 11--12 years, but vaccination at age 14--16 years was also acceptable (29). In 1996, ACIP, in collaboration with partner organizations, recommended a routine vaccination visit at age 11--12 years to reduce adolescent morbidity associated with vaccine-preventable diseases and to improve vaccine coverage for adolescents (30). The 1996 ACIP statement emphasized that the recommended age for Td administration was 11--12 years, if at least 5 years had elapsed since administration of the last pediatric DTP/DTaP dose. Td also was recommended for older adolescents who missed the Td dose at age 11--12 years. In some states, school attendance laws continue to require that adolescents receive the Td dose 10 years after the last tetanus and diphtheria toxoids--containing vaccine, rather than at age 11--12 years (CDC, unpublished data, 2005). After the adolescent Td booster dose, ACIP has recommended Td boosters every 10 years throughout life (24,31). Licensure of Pertussis Vaccines for Use in Adolescents and Adults in the United StatesIn spring 2005, two tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) products were licensed in the United States for use in adolescents (and, for one product, use in adults) (32,33). The pertussis antigen composition of the adolescent and adult Tdap formulations is similar to pediatric DTaP, but some of the pertussis antigens are reduced in quantity. The tetanus and diphtheria toxoid composition of Tdap is similar to licensed adult formulations of Td (Appendix A). No preparation containing pertussis antigens alone is licensed in the United States. Acellular pertussis vaccines formulated for adolescents and adults have been available for use in other countries, including Canada, Australia, and several European countries (e.g., France, Austria and Germany) (10,34--40). Background: PertussisGeneral Clinical CharacteristicsPertussis is an acute respiratory infection caused by Bordetella pertussis, a fastidious gram-negative coccobacillus. The organism elaborates toxins that damage respiratory epithelial tissue and have systemic effects, including promotion of lymphocytosis (41,42). Other species of Bordetella, including B. parapertussis, and less commonly B. bronchiseptica or B. holmesii, are associated with cough illness; the clinical presentation of B. parapertussis can be similar to that of classic pertussis. Illnesses caused by species of Bordetella other than B. pertussis are not preventable by pertussis vaccines (43). Factors that affect the clinical presentation of pertussis include age, the level of immunity, and use of antimicrobials early in the course of the illness (44). The usual incubation period for pertussis is 7--10 days (range: 5--21 days) (20,44,45). Classic pertussis is characterized by three phases of illness: catarrhal, paroxysmal, and convalescent (20,44,45). During the catarrhal phase, which generally lasts 1--2 weeks, infected persons experience coryza and an intermittent cough; high fever is uncommon. The paroxysmal phase usually lasts 4--6 weeks and is characterized by spasmodic cough, posttussive vomiting, and inspiratory whoop. Absolute lymphocytosis is common in unvaccinated children (46). Symptoms slowly improve during the convalescent phase, which generally lasts 2--6 weeks, but can last months. Complications can occur during the course of pertussis, including hypoxia, pneumonia, weight loss, seizures, encephalopathy, and death (20,41,44,47). Infants aged <12 months with pertussis are more likely than older age groups to have complications or be hospitalized during their illness (16,47,48). During 2000--2004, an average of 2,488 cases of pertussis was reported annually among infants aged <12 months. Among these infants, 63% were hospitalized, and the median duration of hospitalization was 5 days (range: 1--152 days) (CDC, unpublished data, 2005). Two to 3 doses of pediatric DTaP (recommended at ages 2, 4, and 6 months) provide protection against severe pertussis (16,48,49). Young infants, who can present with symptoms of apnea and bradycardia without cough, are at highest risk for death from pertussis (16,47,48). During 1980--2004, a total of 223 pertussis-related deaths in infants aged <4 months were reported to CDC (of 280 in all age groups) (48, CDC, unpublished data, 2005). Of the 100 pertussis-related deaths reported during 2000--2004, a total of 90 (90%) were among young infants aged <4 months and 76 (76%) were among infants aged <2 months (CDC, unpublished data, 2005). B. pertussis is primarily transmitted from person to person through large respiratory droplets generated by coughing or sneezing. Persons with pertussis are most infectious during the catarrhal and early paroxysmal phases of illness (20,50). The disease is highly communicable, with attack rates as high as 80%--90% among nonimmune household contacts (20,24,44). Adolescents with pertussis can transmit the disease to infants. A study conducted using enhanced pertussis surveillance during 1999--2002 investigated the source of pertussis among infants aged <12 months. On the basis of parental interview, a source was identified among 264 (43%) of 616 infant cases. An adolescent (defined in the study as a person aged 10--19 years) was identified as the source for 43 (7%) of the 616 infants (51). Clinical Features and Morbidity Among Adolescents with PertussisThe spectrum of disease caused by B. pertussis in adolescents ranges from mild cough illness to classic pertussis; infection also can be asymptomatic. When presentation is not classic, pertussis can be clinically indistinguishable from other respiratory illnesses. Adolescents reported with pertussis commonly experience a prolonged cough illness and sometimes have complications (Table 1); rates of certain clinical characteristics and complications in these types of studies probably are higher than among all adolescents with pertussis because the cases with a more classic presentation are more likely to be diagnosed and reported (52--54; CDC, unpublished data, 2005). Complications and hospitalizations related to pertussis occur in up to 2% of adolescents reported with pertussis (52,54; CDC, unpublished data, 2005). Pertussis-related deaths are rarely reported among adolescents; in the United States during 1990--2004, two pertussis-related deaths among adolescents aged 11--18 years were reported to CDC (one adolescent with malignancy and one adolescent with severe neurologic impairment) (CDC, unpublished data, 2005). A prolonged cough is a common feature of pertussis in adolescents. A study in Quebec, Canada, indicated that 97% of adolescents with pertussis coughed for >3 weeks, and 47% coughed for >9 weeks (52). Massachusetts surveillance data demonstrated that 38% of adolescents with pertussis reported during 1989--2004 had already been coughing for >1 month at the time of diagnosis (Massachusetts Department of Public Health [MDPH], unpublished data, 2005). Adolescents with pertussis often make repeated visits for medical care. Of approximately 7,000 Massachusetts adolescents with pertussis reported during 1989--2004, 41% had one, 32% had two, and 24% had three or more medical visits during their illness (MDPH, unpublished data, 2005). Adolescents with pertussis and their household contacts frequently miss school or work. Of Massachusetts adolescents with pertussis, 83% missed school (mean: 5.5 days; range: 0.4--32 days). In 43% of households with an affected adolescent, one parent or caretaker missed work (mean: 2.4 days, range: 0.1--25), and in 14% of households, a second parent or caretaker missed work (mean: 1.8 days, range: 0.1--11 days) (54). Pertussis DiagnosisMany factors affect the sensitivity, specificity, and interpretation of diagnostic tests for B. pertussis, including the stage of the disease, antimicrobial administration, previous vaccination, the quality of technique used to collect the specimen, transport conditions to the testing laboratory, experience of the laboratory, contamination of the sample, and use of nonstandardized tests (55,56). In addition, tests and specimen collection materials might not be readily available to practicing clinicians. Isolation of B. pertussis by culture is 100% specific; however, sensitivity of culture varies because fastidious growth requirements make it difficult to transport and isolate the organism. Although the sensitivity of culture can reach 80%--90% under optimal conditions, in practice, sensitivity typically ranges from 30%--60% (57). The yield of B. pertussis from culture declines in specimens taken after 2 or more weeks of cough illness, after antimicrobial treatment, or after previous pertussis vaccination (58). Within 3 weeks after onset of cough, culture is only 1%--3% sensitive (59). Although B. pertussis can be isolated in culture as early as 72 hours after plating, it takes 1--2 weeks before a culture result can definitively be called negative (60). Culture is essential to isolate B. pertussis for antimicrobial susceptibility testing and for molecular subtyping of strains. Direct fluorescent antibody (DFA) tests provide rapid results (hours), but are generally less sensitive (sensitivity: 10%--50%) than culture. With use of monoclonal reagents, the specificity of DFA should generally be >90%; however, the interpretation of the test is subjective, and interpretation by an inexperienced microbiologist can result in lower specificity (61). Because of the limitations of DFA testing, CDC does not recommend its use. Because of increased sensitivity and shorter turn-around-time, DNA amplification (e.g., polymerase chain reaction [PCR]) is being used more frequently to detect B. pertussis. When symptoms of classic pertussis are present (e.g., 2 weeks of paroxysmal cough), PCR typically is 2--3 times more likely than culture to detect a positive B. pertussis sample (56,62,63). The interpretation of PCR-positive but culture-negative samples as either true positive or false positive is difficult. No U.S. Food and Drug Administration (FDA)--licensed PCR test kit and no national standardized protocols, reagents, and reporting formats are available. Approximately 100 different PCR protocols have been reported. These vary by DNA purification techniques, PCR primers, reaction conditions, and product detection methods (63). Laboratories must develop and validate their own PCR tests. As a result, the analytical sensitivity, accuracy, and quality control of PCR-based B. pertussis tests might vary widely among laboratories. The majority of laboratory validation studies have not sufficiently established the predictive value of a positive PCR test to diagnose pertussis (63). Use of PCR tests with low specificity can result in unnecessary investigation and treatment of persons with false-positive PCR test results and inappropriate chemoprophylaxis of their contacts (63). CDC Council of State and Territorial Epidemiologists (CSTE) reporting guidelines support the use of PCR to confirm the diagnosis of pertussis only when the case also meets the clinical case definition (>2 weeks of cough with paroxysms, inspiratory "whoop," or posttussive vomiting) (Appendix B) (64,65). Diagnosis of pertussis by serology generally requires demonstration of a substantial change in titer for pertussis antigens (usually fourfold) when comparing results from acute (<2 weeks after cough onset) and convalescent sera (>4 weeks after the acute sample). The results of serologic tests on paired sera generally become available late in the course of illness and can provide only retrospective diagnosis. A single sample serologic assay with age-specific antibody reference values is used as a diagnostic test for adolescents and adults in Massachusetts but is not available elsewhere (66). Other single-sample serologic assays lack standardization and do not clearly differentiate immune responses to pertussis antigens after recent clinical disease, from more remote disease, or from vaccination (43). None of these serologic assays, including the Massachusetts assay, is licensed by FDA for routine diagnostic use in the United States. For these reasons, CDC guidelines for laboratory confirmation of pertussis cases do not include serologic testing. The only pertussis diagnostic tests that the CDC endorses are culture and PCR (when the CDC/CSTE clinical case definition is also met) (Appendix B). CDC-sponsored studies are underway to evaluate both serology and PCR testing. CDC guidance on the use of pertussis diagnostics will be updated as results of these studies become available. Incidence of Pertussis Among AdolescentsPertussis is reportable in all 50 states and the District of Columbia. State health departments report confirmed and probable cases of pertussis to CDC through the passive National Notifiable Diseases Surveillance System (NNDSS); additional information for pertussis cases is collected through the Supplemental Pertussis Surveillance System (SPSS) (Appendix B) (4,16). During 2004, a total of 8,897 (34%) of the 25,827 reported U.S. cases occurred among adolescents aged 11--18 years (incidence for adolescents: 30 per 100,000 population); 17 states each reported >100 pertussis cases in adolescents (12, CDC unpublished data, 2005*). The age distribution of the other pertussis cases reported in 2004 was 3,357 (13%) among infants aged <1 years, 5,441 (21%) among children aged 1--10 years, and 7,481 (29%) among adults aged >19 years (the age was unknown for 2.5% of the cases). The incidence of pertussis in adolescents varies widely among states and from year-to-year. During 2000--2004, a total of 11 states had an annual incidence of reported pertussis in adolescents of >50 per 100,000 population during at least 1 year (Table 2) (12, CDC, unpublished data, 2005). Data from enhanced surveillance sites and prospective studies indicate that the national passive surveillance data substantially underestimate the burden of pertussis among adolescents. Reliable diagnostic tests are not widely available, and not all diagnosed cases are reported. Since the 1980s, MDPH has conducted enhanced surveillance for pertussis throughout Massachusetts. MDPH uses an in-state, standardized serologic assay for pertussis diagnosis in adolescents and adults; educates health-care providers, public health staff, and the general public about pertussis; and intensifies surveillance around cases, particularly in school settings (17,66; MDPH, unpublished data, 2005). During 1996--2004, the average annual incidence of pertussis in Massachusetts adolescents aged 11--18 years was 93 per 100,000 population, approximately 13 times greater than the incidence of 7.3 reported for adolescents in the remainder of the United States (Figure 2); reported rates among children aged <11 years were comparable between Massachusetts and the remainder of the United States (CDC, unpublished data, 2005). Massachusetts data indicated that 62% of reported pertussis cases in adolescents occurred before age 16 years, and 28% of reported cases occurred before age 14 years, suggesting that pertussis booster vaccination early in adolescence could have a substantial impact on the burden of pertussis in adolescents (Figure 3) (CDC, unpublished data, 2005). Two prospective studies in the United States have assessed the incidence of pertussis in populations that included adolescents (67,68). In a Minnesota health maintenance organization during 1995--1996, persons aged 10--49 years were tested for pertussis if they presented with an acute paroxysmal cough or a persistent cough illness of 7--34 days duration (67). Cases were laboratory-confirmed using culture, PCR, and/or serology. On the basis of 27 identified cases, the estimated incidence of laboratory-confirmed pertussis in this study was 507 per 100,000 person-years, and the incidence in adolescents (estimated 997 per 100,000 person-years) was the highest of the age groups studied (67). During 1997--2000, a study conducted at sites in eight states estimated the incidence of pertussis among persons aged 15--64 years enrolled in the control arm of an acellular pertussis vaccine trial (68). The incidence of pertussis, defined as an acute cough illness of at least 5 days with laboratory confirmation, by culture, PCR, and/or serology, was 370 per 100,000 person-years. Applying less specific case definitions, the study suggested approximately 1,000,000 cases of pertussis occur annually among persons aged >15 years in the United States (68). Pertussis Outbreaks Involving AdolescentsHigh rates of pertussis in adolescents have been reported during community and statewide outbreaks. For example, in a 1985 outbreak that occurred in a three-county region in semirural central Wisconsin, 32% of 161 cases of pertussis occurred in adolescents aged 10--19 years for an incidence (using only culture-positive cases, the strictest case definition for confirmed cases) of 150 per 100,000 population during the 8-month outbreak period (69). Compared with the incidence of pertussis in other age groups, the incidence of pertussis in adolescents was second only to that in infants aged <1 year (496 per 100,000 population) (69). In a statewide 1996 outbreak in Vermont, younger adolescents aged 10--14 years accounted for 36% of 280 cases and had the highest incidence (235 per 100,000 population) of all age groups during the outbreak period (70). Reported cases of pertussis in adolescents often occur in outbreaks at middle and high schools, where close interaction occurs among large numbers of students with waning vaccine-induced immunity to pertussis. Delay in the diagnosis of pertussis contributes to the spread of pertussis in schools. Although the actual number of pertussis outbreaks in schools across the United States is unknown, descriptions of pertussis outbreaks in middle and high schools from several states suggest that these outbreaks are not uncommon. Surveillance data from Massachusetts during 2000--2004 indicated that approximately 90% of the detected pertussis outbreaks (defined in Massachusetts as five or more cases linked in location and time) occurred in schools. During this period, 41% of the reported pertussis cases in adolescents aged 11--19 years were identified through school outbreaks (MDPH, unpublished data, 2005) (Table 3). Middle and high school outbreaks of pertussis can disrupt usual school functions and result in substantial public health and school efforts to educate families, detect and treat cases, and provide chemoprophylaxis to close contacts. For example, in 2001 in Pike County, Arkansas (population: 11,222), an outbreak of pertussis began among members of a school football team (71; CDC, unpublished data, 2005). Among the 242 students in the county's middle and high school, 77 cases (attack rate: 32%) occurred, and the school was temporarily closed because of absenteeism. Of the students in the school, 93% had received at least 4 doses of pediatric DTP/DTaP during childhood. Countywide, 140 cases occurred in 109 households; 64% of the cases were reported among adolescents aged 12--18 years (71; CDC, unpublished data, 2005). In July 2003, in Fond du Lac County, Wisconsin (population: 97,296), initial detection of a pertussis outbreak occurred primarily among students who used a high school weight room (72; Wisconsin Division of Public Health, unpublished data, 2005). Of the 313 pertussis cases detected in the county over the 8-month outbreak period, 220 (70%) occurred among adolescents aged 10--19 years for an incidence of 1,505 per 100,000 population. A total of 92 cases occurred in six middle schools (range: 1--37 cases per school); 76 cases occurred among high school students (69 cases in one high school). An estimated 5,000 courses of antimicrobials were prescribed during this pertussis outbreak (72; Wisconsin Division of Public Health, unpublished data, 2005). Background: Tetanus and DiphtheriaTetanusTetanus is unique among diseases for which vaccination is routinely recommended in that it is noncommunicable. Clostridium tetani spores are ubiquitous in the environment and enter the body through nonintact skin. When inoculated into oxygen-poor sites, such as necrotic tissue that can result from blunt trauma or deep puncture wounds, C. tetani spores germinate to vegetative bacilli that multiply and elaborate tetanospasmin, a potent neurotoxin. Generalized tetanus typically presents with trismus (lockjaw), followed by generalized rigidity caused by painful contractions of the skeletal muscles that can impair respiratory function. Glottic spasm, respiratory failure, and autonomic instability can result in death (73). During 1998--2000, the case-fatality ratio for reported tetanus was 18% in the United States (74). Following widespread use of tetanus toxoid--containing vaccine during the 1940s, tetanus has become uncommon in the United States, particularly in children and adolescents (73,75). During 1990--2004, a total of 624 tetanus cases were reported; 19 (3%) cases were among adolescents aged 11--18 years (76; CDC, unpublished data, 2005). A 3-dose primary series of tetanus toxoid--containing vaccine generally induces protective levels of antibody for tetanus that persist for >10 years (73). Seroprotective rates for tetanus, defined as an antitetanus concentration >0.15 IU/mL (international units/milliliter), were obtained from a population-based national serosurvey (National Health and Nutritional Examination Survey [NHANES] III) conducted in the United States during 1988--1994. NHANES III was conducted when Td vaccination was recommended at age 14--16 years, before routine Td vaccination at age 11--12 years was implemented. Results of NHANES III indicated approximately 80% of adolescents aged 12--19 years had protective antitetanus concentrations (77). Neonatal tetanus usually occurs as the result of C. tetani infection of the umbilical stump of an infant born to a mother with a maternal antitetanus concentration insufficient to provide protection to the infant (73). Neonatal tetanus is extremely rare in the United States: three cases were reported during 1990--2004. Two of the cases occurred among children born to mothers who had no dose or 1 dose of a tetanus toxoid--containing vaccine, and the vaccination history of the third mother was unknown (78,79; CDC, unpublished data, 2005). DiphtheriaRespiratory diphtheria is an acute and communicable infectious illness caused by toxigenic strains of Corynebacterium diphtheriae, and rarely by toxin-producing C. ulcerans; disease is prevented by vaccination with diphtheria toxoid--containing vaccines. Respiratory diphtheria is characterized by a grayish-colored adherent membrane in pharynx, palate, or nasal mucosa that can obstruct the airway. In addition, toxin-mediated cardiac and neurologic complications can occur (80,81). Reports of respiratory diphtheria are rare in the United States in all age groups (80,82). During 1998--2004, seven cases of respiratory diphtheria were reported to CDC; one of the cases was imported (11,12). The last culture-confirmed case of respiratory diphtheria in a U.S. adolescent was reported in 1996 (82). Data obtained from the NHANES III serosurvey during 1988--1994 indicated that the prevalence of immunity to diphtheria, defined as an antidiphtheria concentration of >0.1 IU/mL, was approximately 80% among adolescents aged 12--19 years (77). Exposure to diphtheria remains possible during travel to countries where diphtheria is endemic (information available at http://www.cdc.gov/travel/diseases/dtp.htm) or from imported cases. Respiratory diphtheria also can occur following exposure to toxin-producing strains of C. ulcerans; some cases have followed contact with dairy animals or consumption of unpasteurized dairy products (80,83). Adherence to the ACIP-recommended schedule for tetanus and diphtheria toxoid--containing boosters among adolescents and adults is important to prevent sporadic cases of respiratory diphtheria. Information about the clinical management of diphtheria, including use of diphtheria antitoxin, and the public health response is available at http://www.cdc.gov/nip/vaccine/dat/default.htm and reviewed elsewhere (24,80,84). Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccines for AdolescentsCriteria for Tdap LicensureIn the United States, two Tdap products were licensed for use in adolescents and, for one product, use in adults as a single dose booster immunization against tetanus, diphtheria, and pertussis on the basis of clinical trials demonstrating immunogenicity not inferior to U.S.- licensed Td or pediatric DTaP products and an overall safety profile clinically comparable to U.S.-licensed Td products (85,86). In a noninferiority trial, immunogenicity, efficacy, or safety endpoints are demonstrated when a new product is at least as good as a comparator on the basis of a predefined and narrow margin for a clinically acceptable difference between the study groups (87). The efficacy of the tetanus and diphtheria toxoid component of each Tdap was based on the immunogenicity of these antigens compared with U.S.-licensed Td using established serologic correlates of protection (73,81). The percentage of persons achieving seroprotective antitetanus and antidiphtheria concentrations (>0.1 IU/mL) and the booster response to each of these antigens 1 month postvaccination were evaluated. In contrast to tetanus and diphtheria, no well-accepted serologic or laboratory correlate of protection for pertussis exists (88). A consensus was reached at the 1997 meeting of the Vaccines and Related Biological Products Advisory Committee that clinical endpoint efficacy studies of acellular pertussis vaccines among adolescents or adults were not required for Tdap licensure in these age groups. Rather, the efficacy of the pertussis components of Tdap administered to adolescents and adults could be inferred using a serologic bridge to infants vaccinated with pediatric DTaP during clinical endpoint efficacy trials for pertussis (89). For each Tdap product, the immune response (geometric mean antibody concentration [GMC]) of adolescents to each vaccine pertussis antigen after a single dose of Tdap was compared with the immune response of infants after three doses of pediatric DTaP that included the same pertussis components as the Tdap being assessed (32,33). The percentage of adolescents with a booster response to vaccine pertussis antigens exceeding a predefined lower limit for an acceptable booster response also was evaluated. The safety of Tdap was evaluated by comparing rates of adverse events after vaccination in persons receiving Tdap with those receiving Td. The overall safety profile of Tdap also was assessed. Tdap Product InformationData on immunogenicity and safety for the Tdap products licensed in the United States for use in adolescents (and, for one product, use in adults) are presented separately below. BOOSTRIX® Indication BOOSTRIX®, Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed (Tdap) (GlaxoSmithKline Biologicals [GSK], Rixensart, Belgium), was licensed on May 3, 2005, for active booster immunization against tetanus, diphtheria, and pertussis as a single dose in persons aged 10--18 years (33). Vaccine Composition BOOSTRIX® contains the same tetanus toxoid, diphtheria toxoid, and three pertussis antigens (inactivated pertussis toxin [PT], formaldehyde-treated filamentous hemagglutinin [FHA] and pertactin [69 kiloDalton outer membrane protein] [PRN]), as those in INFANRIX® (pediatric DTaP), but BOOSTRIX® is formulated with reduced quantities of these antigens (Appendix A). Each antigen is adsorbed onto aluminum hydroxide. Each dose of BOOSTRIX® (0.5 mL) is formulated to contain 5 Lf [limit of flocculation unit] tetanus toxoid, 2.5 Lf diphtheria toxoid, 8 µg inactivated PT, 8 µg FHA and 2.5 µg PRN. Each dose of BOOSTRIX® also contains aluminum hydroxide (<0.39 mg aluminum) as the adjuvant, 4.5 mg NaCl, <100 µg residual formaldehyde, and <100 µg polysorbate 80 (Tween 80) per 0.5--mL dose. BOOSTRIX® contains no thimerosal or other preservative. BOOSTRIX® is available in two presentations: a prefilled disposable syringe without a needle and a single dose vial. The tip cap and rubber plunger of the needleless prefilled syringe contain dry natural latex rubber; the single dose vial stopper preparation is latex-free (33). Immunogenicity and Serologic Bridge to Efficacy for Pertussis A comparative, observer-blinded, multicenter, randomized controlled clinical trial conducted in the United States evaluated the immunogenicity of the tetanus, diphtheria, and pertussis antigens in BOOSTRIX® among adolescents aged 10--18 years (33,85). Adolescents were randomized 3:1 to receive a single dose of BOOSTRIX® or a single dose of TdMPHBL (manufactured by the Massachusetts Public Health Biologic Laboratories; contains diphtheria toxoid [2 Lf] and tetanus toxoid [2 Lf]) (33,85). Serum samples were obtained before and approximately 1 month after vaccination (85). All assays were performed by GlaxoSmithKline Biologicals in Rixensart, Belgium, using validated methods (90). Persons were eligible for enrollment if they were in good health and had completed routine childhood vaccination against diphtheria, tetanus, and pertussis (approximately 98% of persons had received >4 doses of pediatric DTP/DTaP). Persons were excluded if they had received the most recent pediatric DTP/DTaP during the preceding 5 years or Td during the preceding 10 years, had a history of pertussis or household exposure to pertussis during the previous 5 years, had any of the ACIP contraindications or precautions for pediatric DTP/DTaP (91), had systemic allergic or neurologic reactions or thrombocytopenia after a dose of tetanus or diphtheria toxoid--containing vaccine, had an acute illness, had received blood products or immunoglobulins within 3 months, had any immunodeficiency, had significant underlying disease, had certain neurologic disorders, or were pregnant (33,85,92; GSK, unpublished data, 2005). Tetanus and Diphtheria Immune responses to tetanus and diphtheria toxoids were compared between the BOOSTRIX® (range: 2,463--2,516 persons) and TdMPHBL (range: 814--834 persons) groups. One month postvaccination, the antitetanus seroprotective (>0.1 IU/mL) and booster response rates in adolescents who had received a single dose of BOOSTRIX® were noninferior to those who received TdMPHBL. All adolescents had seroprotective antitetanus levels >0.1 IU/mL 1 month after vaccination with either BOOSTRIX® (95% confidence interval [CI] = 99.8%--100%) or TdMPHBL (95% CI = 99.6%--100%). The booster response rate to tetanus† in the BOOSTRIX® group was 89.7% (95% CI = 88.4%--90.8%), compared with 92.5% (95% CI = 90.5%--94.2%) in the TdMPHBL group (33,92). One month postvaccination, the antidiphtheria seroprotective (>0.1 IU/mL) and booster response rates among adolescents who received a single dose of BOOSTRIX® were noninferior to those of adolescents who received TdMPHBL. Among adolescents, 99.9% had seroprotective antidiphtheria levels >0.1 IU/mL 1 month postvaccination with either BOOSTRIX® (95% CI = 99.7%--100%) or TdMPHBL (95% CI = 99.3%--100%). The booster response rate to diphtheria† in the BOOSTRIX® group was 90.6% (95% CI = 89.4%--91.7%), compared with 95.9% (95% CI = 94.4%--97.2%) in the TdMPHBL group (33,92). Pertussis The efficacy of the pertussis components of BOOSTRIX® was evaluated by comparing the immune responses of adolescents vaccinated with a single dose of BOOSTRIX® with the immune responses of infants vaccinated with 3 doses of INFANRIX®. These infants were a subset of those vaccinated with INFANRIX® in a German vaccine efficacy trial during the 1990s (33,93). BOOSTRIX® has the same three pertussis antigens as INFANRIX® but in reduced quantities (Appendix A). In the infant trial, the efficacy of 3 doses of INFANRIX® against World Health Organization (WHO)-defined typical pertussis (>21 days of paroxysmal cough with confirmation of B. pertussis infection by culture and/or serologic testing) was 89% (95% CI = 77%--95%) (33,93). The anti-PT, anti-FHA, and anti-PRN GMCs of adolescents 1 month after a single dose of BOOSTRIX® were noninferior to those of infants after 3 doses of INFANRIX® (Table 4) (33,85,92). Booster response rates to the pertussis antigens§ contained in BOOSTRIX® (anti-PT, anti-FHA, and anti-PRN) among adolescents (range: 2,677--2,752 persons) 1 month after administration of BOOSTRIX® met prespecified criteria for an acceptable response. Booster response rates to pertussis antigens were anti-PT, 84.5% (95% CI = 83.0%--85.9%); anti-FHA, 95.1% (95% CI = 94.2%--95.9%), and anti-PRN, 95.4% (95% CI = 94.5%--96.1%) (33,92). Safety The primary safety study, conducted in the United States, was a randomized, observer-blinded, controlled study in which 3,080 adolescents aged 10--18 years received a single dose of BOOSTRIX®, and 1,034 received TdMPHBL (see BOOSTRIX® Immunogenicity and Serologic Bridge to Efficacy for Pertussis for inclusion and exclusion criteria). Data on solicited local and systemic adverse events were collected using standardized diaries for the day of vaccination and the next 14 consecutive days (i.e., within 15 days following vaccination). Unsolicited and serious adverse events were collected for 6 months following vaccination. No immediate events (within 30 minutes of vaccination) were reported in either vaccination group (33,85,92). Solicited Local Adverse Events Pain at the injection site was the most frequently reported solicited local adverse event in adolescents vaccinated with BOOSTRIX® or TdMPHBL. Within 15 days after vaccination, 75.3% of persons in the BOOSTRIX® group and 71.7% of persons in the TdMPHBL group reported pain of "any" intensity (Table 5). The rates of any pain and grade 2 or 3 pain combined (but not grade 3 alone) were significantly higher (p<0.05) in BOOSTRIX® recipients compared with TdMPHBL recipients (Table 5). However, the rates of grade 3 pain (primary safety endpoint) were similar in each group, and the noninferiority criterion was met for BOOSTRIX® compared with TdMPHBL. No significant differences in the rates of other solicited local adverse events (redness, swelling, and increase in arm circumference above baseline) were observed between the two study groups (33,85,92). Two adolescents in the study reported "large injection-site swelling" after vaccination (predefined as any local swelling with a diameter >100 mm and/or increased circumference of the injected limb >50 mm above baseline measurements and/or any diffuse swelling that interfered with or prevented normal everyday activities). Both persons had onset of symptoms within 3 days of vaccination. One person who had received BOOSTRIX® reported grade 3 pain (Table 5) with functional impairment. This person was evaluated and treated with antimicrobials with symptom resolution within 3 days without sequelae. The second person, who had received TdMPHBL, reported grade 1 pain and did not seek medical attention. The duration of symptoms was unknown, but symptoms resolved without sequelae (85,90,92). No cases of whole-arm swelling were reported in either vaccine group (GSK, unpublished data, 2005). Solicited Systemic Adverse Events The most frequently reported solicited systemic adverse events within 15 days following vaccination with BOOSTRIX® or TdMPHBL were headache and fatigue (Table 6). A statistically significantly higher rate of grade 2 or grade 3 headache combined (but not grade 3 alone) (Table 6) was reported in the BOOSTRIX® group (15.7%), compared with the TdMPHBL group (12.7%). The proportion of adolescents reporting fever >100.4° F (38.0° C) (5.0% for BOOSTRIX® and 4.7% for TdMPHBL), fatigue, and gastrointestinal systemic events were comparable in both groups (33,85,92). Serious Adverse Events In the primary U.S. safety study, no serious adverse events (SAEs) occurred within 1 month postvaccination with either BOOSTRIX® or TdMPHBL. During the next 5 months of monitoring, SAEs were reported among 14 (0.4%) of the 3,005 adolescents vaccinated with BOOSTRIX® and two (0.2%) of the 1,003 adolescents vaccinated with TdMPHBL. No SAEs that were of potential autoimmune origin, new onset and chronic in nature, or related to vaccination, as determined by the investigators, were reported (33,85,90,92). No seizures, cases of Guillain-Barré syndrome, or physician-diagnosed Arthus reactions were reported (33,85,90; GSK, unpublished data, 2005). Simultaneous Administration with other Vaccines Safety and immunogenicity of simultaneous administration of BOOSTRIX® with other vaccines were not evaluated during prelicensure studies (33). ADACEL™ Indication ADACEL™, Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed (Tdap) (sanofi pasteur, Toronto, Ontario, Canada) was licensed on June 10, 2005, for active booster immunization against tetanus, diphtheria, and pertussis as a single dose in persons aged 11--64 years (32). Vaccine Composition ADACEL™ contains the same tetanus toxoid, diphtheria toxoid, and five pertussis antigens as those in DAPTACEL® (pediatric DTaP), but ADACEL™ is formulated with reduced quantities of diphtheria toxoid and detoxified PT (Appendix A). Each antigen is adsorbed onto aluminum phosphate. Each dose of ADACEL™ (0.5 mL) is formulated to contain 5 Lf tetanus toxoid, 2 Lf diphtheria toxoid, 2.5 µg detoxified PT, 5 µg FHA, 3 µg PRN, and 5 µg fimbriae types 2 and 3 (FIM). Each dose of ADACEL™ also contains aluminum phosphate (0.33 mg aluminum) as the adjuvant, <5 µg residual formaldehyde, <50 ng residual glutaraldehyde, and 3.3 mg 2-phenoxyethanol (not as a preservative) per 0.5--mL dose. ADACEL™ contains no thimerosal. ADACEL™ is available in single dose vials that are latex-free (32). Immunogenicity and Serologic Bridge to Efficacy for Pertussis A comparative, observer-blinded, multicenter, randomized controlled clinical trial conducted in the United States evaluated the immunogenicity of the tetanus, diphtheria, and pertussis antigens in ADACEL™ among adolescents aged 11--17 years; adults aged 18--64 years were also studied and results are reported elsewhere (32,86). Adolescents were randomized 3:2 to receive a single dose of ADACEL™ or a single dose of Tdsp (manufactured by sanofi pasteur; contains tetanus toxoid [5 Lf] and diphtheria toxoid [2 Lf]) (32,86). Sera from a subset of persons were obtained before and approximately 1 month after vaccination (32). All assays were performed at the immunology laboratories of sanofi pasteur in Toronto, Ontario, Canada or Swiftwater, Pennsylvania, using validated methods (86,94). Persons were eligible for enrollment if they were in good health; completion of the childhood DTP/DTaP vaccination series was not required. Persons were excluded if they had received a tetanus, diphtheria, or pertussis vaccine within 5 years; had a diagnosis of pertussis within 2 years; had an allergy or sensitivity to any vaccine component; had a previous reaction to a tetanus, diphtheria or pertussis vaccine, including encephalopathy within 7 days or seizures within 3 days; had an acute respiratory illness on the day of enrollment; had daily use of oral, nonsteroidal anti-inflammatory drugs; had received blood products or immunoglobulins within 3 months; had any immunodeficiency; had significant underlying disease; had neurologic impairment; or were pregnant (32,94; sanofi pasteur, unpublished data, 2005). Tetanus and Diphtheria Immune responses to tetanus and diphtheria toxoids were compared between the ADACEL™ (N = 527) and Tdsp (range: 515--516 persons) groups. One month postvaccination, the antitetanus seroprotective (>0.1 IU/mL) and booster response rates among adolescents who received ADACEL™ were noninferior to those who received Tdsp. All adolescents (95% CI = 99.3%--100% for both groups) had seroprotective antitetanus levels >0.1 IU/mL 1 month after vaccination with either ADACEL™ or Tdsp. The booster response rate to tetanus¶ in the ADACEL™ group was 91.7% (95% CI = 89.0%--93.9%) and 91.3% (95% CI = 88.5%--93.6%) in the Tdsp group (32,86,94). One month postvaccination, the antidiphtheria seroprotective (>0.1 IU/mL) and booster response rates among adolescents who received a single dose of ADACEL™ were noninferior to those who received Tdsp. Among adolescents, 99.8% (95% CI = 98.9%--100%) had protective antidiphtheria levels >0.1 IU/mL 1 month after vaccination with either ADACEL™ or Tdsp. The booster response rate to diphtheria¶ in the ADACEL™ group was 95.1% (95% CI = 92.9%--96.8%) and 95.0% (95% CI = 92.7%--96.7%) in the Tdsp group (32,86,94). Pertussis The efficacy of the pertussis components of ADACEL™ was evaluated by comparing the immune responses of adolescents vaccinated with a single dose of ADACEL™ with the immune responses of infants vaccinated with 3 doses of DAPTACEL® in a Swedish vaccine efficacy trial during the 1990s (32,95). ADACEL™ and DAPTACEL® contain the same five pertussis antigens, except ADACEL™ contains one fourth the quantity of detoxified PT in DAPTACEL® (96) (Appendix A). In the Swedish trial, efficacy of 3 doses of DAPTACEL® against WHO-defined pertussis (>21 days of paroxysmal cough with confirmation of B. pertussis infection by culture and/or serologic testing or an epidemiologic link to a household member with culture-confirmed pertussis) was 85% (95% CI = 80%--89%) (29,87). The anti-PT, anti-FHA, anti-PRN, and anti-FIM GMCs of adolescents 1 month after a single dose of ADACEL™ were noninferior to those of infants following three doses of DAPTACEL® (32,94) (Table 7). Booster response rates to the pertussis antigens** contained in ADACEL™ (anti-PT, anti-FHA, anti-PRN, anti-FIM) among adolescents (range: 524--526 persons) 1 month following administration of ADACEL™ met prespecified criteria for an acceptable response. Booster response rates to pertussis antigens were anti-PT, 92.0% (95% CI = 89.3%--94.2%); anti-FHA, 85.6% (95% CI = 82.3%--88.4%); anti-PRN, 94.5% (95% CI = 92.2%--96.3%); and anti-FIM 94.9% (95% CI = 92.6%--96.6%) (32,94). Safety The primary adolescent safety study, conducted in the United States, was a randomized, observer-blinded, controlled study in which 1,184 adolescents aged 11--17 years received a single dose of ADACEL™ and 792 received Tdsp (see ADACEL™ Immunogenicity and Serologic Bridge to Efficacy for Pertussis for inclusion and exclusion criteria) (32). Adults aged 18--64 years were also studied; those results are reported elsewhere (32). Data on solicited local and systemic adverse events were collected using standardized diaries for the day of vaccination and the next 14 consecutive days (i.e., within 15 days following vaccination) (32). Immediate Events Eleven adolescents experienced immediate events within 30 minutes of vaccination (ADACEL™, six persons [0.5%] and Tdsp, five persons [0.6%]); all events resolved without sequelae. Immediate events included dizziness, syncope, or vasovagal reactions and pain and erythema at the injection site. No anaphylaxis was reported (86,94,96). Solicited Local Adverse Events Pain at the injection site was the most frequently reported solicited local adverse event among adolescents in both vaccination groups (Table 8). Within 15 days following vaccination, reports of pain of "any" intensity were more common among adolescents vaccinated with ADACEL™ (77.8%) than among those vaccinated with Tdsp (71.0%). The noninferiority criterion was not achieved for the rate of any pain following ADACEL™ compared with the rate following Tdsp. Rates of moderate/severe pain, erythema, and swelling following ADACEL™ were comparable to the rates following Tdsp (32,86,94). No cases of whole-arm swelling were reported in either vaccine group (94). Solicited Systemic Adverse Events The most frequently reported solicited systemic adverse events within the 15 days following vaccination were headache, generalized body aches, and tiredness (Table 9). The proportion of adolescents reporting fever >100.4° F (>38° C) following vaccination was statistically significantly higher among adolescents vaccinated with ADACEL™ (5.0%) than Tdsp (2.7%), but noninferiority criterion for ADACEL™ was achieved. The rates of the other solicited systemic adverse events were comparable between the ADACEL™ and Tdsp groups (32,94). Serious Adverse Events In the primary adolescent safety study, SAEs within 6 months after vaccination were reported among 11 (0.9%) of the 1,184 adolescents aged 11--17 years vaccinated with ADACEL™ and eight (1.0%) of the 792 adolescents vaccinated with Tdsp. Two adolescents (one ADACEL™ recipient and one Tdsp recipient) reported seizure events after vaccination; both persons had a history of seizure disorder. SAEs in adolescents were reported by the study investigators to be unrelated to the study vaccine (94). The safety of ADACEL™ among adults also was studied. In the primary adult safety study, SAEs within 6 months after vaccination were reported among 33 (1.9%) of the 1,752 adults aged 18--64 years vaccinated with ADACEL™ and 11 (1.9%) of the 573 adults vaccinated with Tdsp. Two of these SAEs were neuropathic events in ADACEL™ recipients and were assessed by the investigators as possibly related to the study vaccine; in both cases, the symptoms resolved completely over several days (32,86,94,96). No physician-diagnosed Arthus reactions or cases of Guillain-Barré syndrome were reported in any adolescent or adult (32,86,96; sanofi pasteur, unpublished data, 2005). Simultaneous Administration with Other Vaccines Hepatitis B Vaccine Safety and immunogenicity of ADACEL™ co-administered with hepatitis B vaccine (Recombivax HB®, Merck and Co., Inc., White House Station, New Jersey) was evaluated in adolescents aged 11--14 years using methods similar to the primary ADACEL™ studies. Adolescents were randomized to one of two groups. In one group, ADACEL™ and hepatitis B vaccine were administered simultaneously in different arms (simultaneous group; N = 206). In the other group, ADACEL™ was administered first, followed by hepatitis B vaccine 4--6 weeks later (sequential group; N = 204) (32; sanofi pasteur, unpublished data, 2005). No interference was observed in the immune responses to any of the vaccine antigens when ADACEL™ and hepatitis B vaccine were administered simultaneously or sequentially†† (32). Adverse events were solicited only after ADACEL™ vaccination (not hepatitis B vaccination) (86). Within 15 days of vaccination, the reported rates of injection site pain (at the ADACEL™ site) and fever were comparable when ADACEL™ and hepatitis B vaccine were administered simultaneously or sequentially (Table 10). However, rates of erythema and swelling at the ADACEL™ injection site were higher in the simultaneous group than the sequential group, and noninferiority criteria for simultaneous vaccination were not achieved (Table 10) (86). Swollen and/or sore joints were reported in 22.5% of persons in the simultaneous group and 17.9% of persons in the sequential group. Most joint complaints were mild in intensity and the mean duration was 1.8 days (86). Trivalent Inactivated Influenza Vaccine Safety and immunogenicity of ADACEL™ co-administered with trivalent inactivated influenza vaccine ([TIV] Fluzone®, sanofi pasteur, Swiftwater, Pennsylvania) was evaluated in adults aged 19--64 years using methods similar to the primary ADACEL™ studies (32). The immunogenicity data are presented elsewhere (32). The adults were randomized to one of two groups. In one group, ADACEL™ and TIV were administered simultaneously in different arms (simultaneous group; N = 359). In the other group, TIV was administered first, followed by ADACEL™ 4--6 weeks later (sequential group; N = 361) (32; sanofi pasteur, unpublished data, 2005). Adverse events were solicited only after ADACEL™ vaccination (not TIV) (86). Within 15 days of vaccination, rates of erythema, swelling, and fever were comparable in both vaccination groups. However, the rate of pain at the ADACEL™ injection site was higher in the simultaneous group (66.6%) than the sequential group (60.8%), and did not meet the noninferiority criterion (upper limit of the 95% CI on the difference in percentage of persons [the rate following simultaneous vaccination minus the rate following sequential vaccination] was 13.0%, and the criterion was <10%) (86). Other Vaccines Safety and immunogenicity of simultaneous administration of ADACEL™ with other vaccines were not evaluated during prelicensure studies (32). Studies of Adolescent Acellular Pertussis Vaccine ImpactClinical Efficacy TrialThe efficacy against pertussis of an adolescent and adult acellular pertussis (ap) vaccine with the same pertussis antigens included in BOOSTRIX® (without tetanus and diphtheria toxoids) was evaluated in 2,781 adolescents and adults in a prospective, randomized trial (68). Results of this study were not considered as part of Tdap licensure in the United States (see Criteria for Tdap Licensure). Persons aged 15--64 years were randomized to receive one dose of ap vaccine or hepatitis A vaccine (Havrix®, GlaxoSmithKline Biologicals, Rixensart, Belgium). The primary outcome measure was confirmed pertussis, defined by a cough illness lasting >5 days with laboratory evidence of B. pertussis infection by culture, PCR, and/or serologic testing results (acute and convalescent). Nine persons in the hepatitis A vaccine control group and one person in the ap vaccine group had confirmed pertussis during the study period; vaccine efficacy against confirmed pertussis was 92% (95% CI = 32%--99%) (68). Economic StudiesThe societal costs of pertussis are important, and universally vaccinating adolescents against pertussis is likely to be cost effective. In one study, the economic impact of pertussis among Massachusetts adolescents aged 10--17 years was evaluated using the state's enhanced pertussis surveillance system (54). The mean medical cost per adolescent case of pertussis was an estimated $201 and $256 for mild and severe cases of cough illness, respectively (in 2004 dollars), excluding the cost of providing antimicrobials to close contacts of the case-adolescents (53,54). The largest proportion of this cost was for medical office visits and antimicrobial therapy (54). When indirect, nonmedical costs (e.g., missed time from work for parents of adolescents) were included, total societal cost of an adolescent case of pertussis was $361 and $416 for mild and severe cough illness, respectively (in 2004 dollars) (53,54). Two U.S. economic studies have compared adolescent vaccination with other pertussis vaccination strategies (53,97). Both studies identified a single dose of Tdap during adolescence as the most cost-effective strategy, under different assumptions about pertussis incidence, waning immunity, vaccine efficacy, vaccine coverage, and infant transmission. In the first study, a cost-benefit analysis was conducted to compare seven adolescent and/or adult pertussis vaccination strategies during a 10-year interval (2001--2010), using a single dose of a Tdap. In this analysis, the incidence of pertussis among adolescents and adults was estimated from prospective studies to be 450--507 cases per 100,000 population. Strategies included vaccinating all adolescents aged 10--19 years, vaccinating all persons aged >10 years (i.e., universal adolescent and adult vaccination), vaccinating adolescents and adults aged >15 years that were the primary care-takers of infants, and four other adult vaccination strategies. Among these strategies, vaccinating all adolescents was identified as the most cost-effective strategy. Universal adolescent Tdap vaccination was cost-saving to society when the Tdap vaccine and program costs were <$37 (2002 dollars) per adolescent vaccinated (97). In a second study, six adolescent and/or adult Tdap vaccination strategies were compared by modeling health outcomes over the course of a lifetime for hypothetical cohort of 4 million adolescents (53). Incidence rates of pertussis among adolescents and adults were estimated from Massachusetts surveillance data; baseline estimates were 155 and 11 per 100,000 population for adolescents and adults, respectively. The six strategies included no adolescent or adult vaccination, one-time adolescent vaccination at age 11 years, one-time adult vaccination, adult vaccination with decennial Tdap boosters, adolescent and adult vaccination with decennial Tdap boosters, and postpartum vaccination. The study assumed an incremental increase in Tdap price of $15 compared with Td, with a Tdap vaccination cost of $25 per person vaccinated. Universal adolescent vaccination was the most cost-effective strategy. Vaccinating all adolescents once would cost $1,100 per pertussis case prevented or $20,000 per quality adjusted life year (QALY) saved, both in 2004 dollars. By contrast with the cost-benefit analysis (97), which estimated the incidence of pertussis in adolescents to be approximately 3 times higher, Tdap vaccination was not cost-saving under the second study's baseline assumptions (53). In a sensitivity analysis, results from the second study found that if the incidence of adolescent and adult pertussis was four times the base-case estimates, universal adolescent Tdap vaccination would be cost-saving to society (53). Other Tetanus and Diphtheria Toxoids Vaccine Preparations for AdolescentsFour Td (Tetanus and Diphtheria Toxoids, Adsorbed for Adult Use) vaccines are licensed in the United States for active immunization against tetanus and diphtheria among persons aged >7 years (98--101). Two tetanus toxoid vaccines (TT) also are licensed for use in this age group (102,103). Of the two TT products, one is adsorbed and is licensed for use in situations in which the combined antigen preparation (Td) is not indicated (102). The second TT preparation is a fluid vaccine (not adsorbed) and is indicated only for booster doses, not for primary immunization (103). Although TT is licensed in the United States for persons aged >7 years, Td has been preferred for routine use to provide dual protection against tetanus and diphtheria (24) (Appendix A). Safety Considerations for Adolescent Vaccination with Tdap or TdPrelicensure Tdap studies support the safety of these vaccines (32,33). However, sample sizes were insufficient to detect rare adverse events, a limitation of prelicensure trials. Enrollment criteria excluded persons who had received vaccines containing tetanus toxoid, diphtheria toxoid, or pertussis components during the preceding 5 or 10 years (85,86,92,94). In addition, persons with certain neurologic conditions or events following pediatric DTP/DTaP vaccination were excluded from these studies (85,92,94). Therefore, in making recommendations on the spacing and administration sequence of vaccines containing tetanus toxoid, diphtheria toxoid, and/or pertussis components and on vaccination of adolescents with a history of certain neurologic conditions or adverse events after vaccination, ACIP considered data from a range of pre- and postlicensure studies of Tdap and other vaccines containing these components. Safety data being collected from the Vaccine Adverse Event Reporting System (VAERS) and postlicensure studies will facilitate detection of potential adverse reactions following widespread use of Tdap in adolescents (see Reporting of Adverse Events after Vaccination) (104,105). Spacing and Administration Sequence of Vaccines Containing Tetanus Toxoid, Diphtheria Toxoid, and Pertussis AntigensHistorically, moderate and severe local reactions following tetanus and diphtheria toxoid--containing vaccines have been associated with older, less purified vaccines, larger doses of toxoid, and frequent dosing at short intervals (106--111). In addition, high pre-existing antibody titers to tetanus or diphtheria toxoids in children, adolescents, and adults primed with these antigens might be associated with increased rates of local reactions to booster doses of tetanus or diphtheria toxoid--containing vaccines (26,108,111,112). Two adverse events of particular clinical interest have been associated with vaccines containing tetanus toxoid, diphtheria toxoid, and/or pertussis antigens, extensive limb swelling (ELS), and Arthus reactions. Extensive Limb Swelling ELS reactions have been described following doses of pediatric DTaP and other vaccines (28,91,113--117). In retrospective analyses, 2%--3% of children receiving the fourth or fifth booster doses of pediatric DTaP experienced extensive proximal limb swelling; swelling is usually greatest by 48 hours after vaccination (28,118). ELS is generally not disabling, is not often brought to medical attention, and resolves without complication within 4--7 days (118). ELS has been reported to VAERS almost as frequently following Td as following pediatric DTaP; among adolescents, the majority of reported cases of ELS have involved either Td or hepatitis B vaccine (117). The pathogenesis of ELS is not well understood; this reaction has not consistently been related to the content of tetanus toxoids, diphtheria toxoids, pertussis antigens, or aluminum adjuvants in vaccines (28,118,119). Whether children who experience ELS after receipt of pediatric DTaP are at increased risk for ELS after receipt of Tdap is unknown. Because these reactions typically resolve without sequelae, ACIP does not consider a history of ELS following pediatric DTaP or any other vaccine to be a precaution or contraindication for pediatric DTaP (91,113). Arthus Reactions Arthus reactions (type III hypersensitivity reactions) are rarely reported after vaccination and can occur after tetanus toxoid--containing or diphtheria toxoid--containing vaccines (24,111,120--124; CDC, unpublished data, 2005). An Arthus reaction is a local vasculitis associated with deposition of immune complexes and activation of complement. Immune complexes form in the setting of high local concentration of vaccine antigens and high circulating antibody concentration (111,120,121,125). Arthus reactions are characterized by severe pain, swelling, induration, edema, hemorrhage, and occasionally by necrosis. These symptoms and signs usually occur 4--12 hours after vaccination; by contrast, anaphylaxis (immediate type I hypersensitivity reactions) usually occur within minutes of vaccination. As with ELS, Arthus reactions usually resolve without sequelae. ACIP has recommended that persons who experienced an Arthus reaction after a dose of tetanus toxoid--containing vaccine should not receive Td more frequently than every 10 years, even for tetanus prophylaxis as part of wound management (24). Interval between Td and Tdap ACIP has recommended a 10-year interval for routine administration of Td and a 5-year minimum interval between the last pediatric DTaP and the adolescent Td dose (24,30). Administration of Td at short intervals might increase the risk for adverse events (108,109). Prelicensure clinical trials of Tdap excluded persons who had received doses of a diphtheria or tetanus toxoid--containing vaccine during the preceding 5 or 10 years (see BOOSTRIX® and ADACEL™ sections on Immunogenicity and Serologic Bridge to Efficacy for Pertussis for exclusion criteria) (90,94,96). Although administering Td more often than every 10 years (5 years for a tetanus-prone wound) is not necessary to provide protection against tetanus or diphtheria, administering a dose of Tdap less than 5 years after Td could provide a health benefit by protecting against pertussis. The safety of administering a dose of Tdap at intervals less than 5 years after pediatric DTP/DTaP or Td was evaluated in Canada following the country's licensure of Tdap (ADACEL™) (126). The largest Canadian study was a nonrandomized, open-label study of 7,001 students aged 7--19 years residing in Prince Edward Island. This study assessed the rates of adverse events after Tdap and compared reactogenicity of Tdap administered at year intervals of 2--9 years (eight cohorts) versus >10 years after the last tetanus and diphtheria toxoid--containing vaccine (Td, or pediatric DTP or DTaP). A year interval was defined as the integer year +0.5 years (e.g., the 2-year interval was defined as >18 months to <30 months). Vaccination history for type of pertussis vaccine(s) received (pediatric DTP and DTaP) also was assessed. The number of persons assigned to cohorts ranged from 464 in the 2-year to 925 in the 8-year cohorts. Among the persons in the 2-year cohort, 214 (46%) received the last tetanus and diphtheria toxoid--containing vaccine 18--23 months before Tdap. Adverse event diary cards were returned for 85% of study participants with a known interval; 90% of persons in the 2-year interval cohort provided safety data (126). Four SAEs were reported in the Prince Edward Island study; none were assessed by the investigators to be related to vaccine. No Arthus reaction was reported. Rates of reported severe local adverse reactions, fever, and any pain were not increased in persons who received Tdap at intervals less than 10 years. Rates of local reactions were not increased among persons who received 5 doses of pediatric DTP, with or without Td (intervals of 2--3 years or 8--9 years). Rates of any erythema and any swelling were reported more frequently in cohorts that had received at least 1 or 2 doses of pediatric DTaP (intervals of 4--7 years), suggesting that increased rates of local reactions might occur more commonly among adolescents who received pediatric DTaP vaccines, compared with those who received a 5-dose pediatric DTP series. Limb swelling (>100 mm) was reported in 0.2% of participants and was unrelated to the interval since the last tetanus and diphtheria toxoid--containing vaccine (126). A study was conducted in Germany to evaluate the safety of Tdap (BOOSTRIX®) in persons aged 9--13 years who received a 5-dose all acellular pertussis vaccine schedule (5 doses of INFANRIX®, N = 193 or 4 doses of INFANRIX® plus another acellular pertussis vaccine, N = 126); the interval from the fifth to sixth dose of the acellular pertussis vaccines ranged from approximately 5--6 years (GSK, unpublished data, 2005). Within 15 days after vaccination, any pain (63.6%), erythema (51.7%), and swelling (41.4%) were frequently reported symptoms, but the rate of "large injection site swelling" (see BOOSTRIX®, solicited local adverse events for definition) was low (0.9%). Following their sixth consecutive dose of an acellular pertussis vaccine, persons reported more pain, less redness, and less swelling compared with their fifth dose of pediatric DTaP (INFANRIX®) (127). Two smaller Canadian postlicensure safety studies in adolescents also showed acceptable safety when Tdap (ADACEL™) was administered at intervals less than 5 years after tetanus and diphtheria toxoid--containing vaccines (128,129). Taken together, these three Canadian studies support the safety of using Tdap after Td at intervals less than 5 years. The largest study suggests intervals as short as approximately 2 years are acceptably safe (126). Simultaneous and Nonsimultaneous Vaccination with Tdap/Td and Diphtheria-Containing Tetravalent Meningococcal Conjugate Vaccine Tdap, Td, and tetravalent meningococcal conjugate vaccine ([MCV4] Menactra®, sanofi pasteur, Swiftwater, Pennsylvania) contain diphtheria toxoid (32,33,130,131). Each of these vaccines is licensed for use in adolescents, but MCV4 is not indicated for active immunization against diphtheria (131). During 2005, MCV4 was recommended for routine use among adolescents (130,132). In MCV4, the diphtheria toxoid (approximately 48 µg) serves as the carrier protein that improves immune responses to meningococcal antigens. Precise comparisons cannot be made between the quantity of diphtheria toxoid in the vaccines; however, the amount in a dose of MCV4 is estimated to be comparable with the average quantity in a dose of pediatric DTaP (133). No prelicensure studies of simultaneous or sequential vaccination with Tdap and MCV4 were done. None of the persons in the Canadian safety studies described above had received MCV4. Persons who recently received one diphtheria toxoid--containing vaccine might have increased rates of adverse reactions after a subsequent diphtheria toxoid--containing vaccine when diphtheria antibody titers remain elevated from the previous vaccination (26,108,111,112). The diphtheria GMCs were comparable or lower following Tdap compared with Td§§; therefore, results of a co-administration trial of Td and MCV4 might be informative to infer the effect of co-administration of Tdap and MCV4. A randomized, controlled prelicensure trial assessed the safety of simultaneous versus sequential administration of Td and MCV4. In this co-administration trial, administration of Td (approximately 8 µg of diphtheria toxoid) with MCV4 first, then placebo 28 days later (simultaneous group), or administration of Td with placebo first, then MCV4 28 days later (sequential group) was studied among 1,021 healthy adolescents aged 11--17 years (Table 11) (89,131,133). Serum samples from a subset of adolescents vaccinated with MCV4 from a different clinical trial were used for comparison. Administration of MCV4 first followed by Td was not studied. Both simultaneous and sequential administration of Td and MCV4 induced immune responses to all antigens. One month postvaccination, the immune responses to diphtheria toxoid were higher when MCV4 was administered simultaneously with Td (GMT 120.9 IU/mL) than when Td was administered with placebo (GMT 8.4 IU/mL). Adolescents vaccinated with MCV4 alone also had high antidiphtheria responses 1 month following vaccination (GMT 46.5 IU/mL) (Table 11) (89,131,133). Adverse events on vaccination day and during the following 7 days were assessed among adolescents in the simultaneous and sequential groups. The overall rates of solicited local reactions were similar for the two groups: 58.0% reported local reactions at the MCV4 injection site in the simultaneous group versus 57.0% in the sequential group, and 74.7% reported local reactions at the Td injection site in the simultaneous group versus 73.3% in the sequential group. Pain was the most frequently reported reaction and pain rates were similar between groups; severe pain was uncommon (<1% of persons). Fever >99.5° F (>37.5° C) and high fever >104° F (>40° C) were reported in <5% and <0.5% of persons, respectively (133--135). The safety of simultaneous vaccination of MCV4 with Tdap and sequential administration of Tdap first followed by MCV4 1 month later has been inferred from results of this study. When Tdap or Td is administered after MCV4, rates of adverse reactions might be higher than when the dose is administered before MCV4 because pre-existing diphtheria antibody levels might be higher (26,108,111,112,133,135). Rates of adverse reactions were assessed in a small prelicensure study for MCV4 among 76 adolescents aged 15--17 years who received 2 doses of MCV4 spaced 3 years apart. After the second dose of MCV4, no SAEs, fever >102.2° F (>39° C), or severe local reactions were reported with the exception of one person, who reported severe pain at the injection site that lasted for 1 day (sanofi pasteur, unpublished data, 2005). No other available data exist on the safety of intervals between MCV4 and other diphtheria toxoid--containing vaccines. Postlicensure studies to provide additional information are under way (104,105). Neurologic and Systemic Events Associated with Vaccines with Pertussis Components or Tetanus Toxoid--Containing VaccinesVaccines with Pertussis Components Concerns about the possible role of vaccines with pertussis components in causing neurologic reactions or exacerbating underlying neurologic conditions are long-standing (20,136). ACIP recommendations to defer pertussis vaccines in infants with suspected or evolving neurologic disease, including seizures, have been based primarily on concerns that neurologic events after vaccination (with whole cell preparations in particular) might interfere with the subsequent evaluation of the infant's neurologic status (1,136). In 1991, the Institute of Medicine (IOM) concluded that evidence favored acceptance of a causal relation between pediatric DTP and acute encephalopathy; the IOM has not evaluated associations between pediatric DTaP and neurologic events (123). Pediatric DTaP is contraindicated for children with a history of encephalopathy not attributable to another identifiable cause occurring within 7 days after pediatric DTP/DTaP vaccination. Though active surveillance in Canada during 1993--2002 failed to ascertain any acute encephalopathy cases causally related to whole cell or acellular pertussis vaccines among a population administered 6.5 million doses of pertussis vaccines (137), research conducted in Japan during the introduction of acellular pertussis vaccines yielded rates of 1.0--1.3 attributable encephalopathy cases within 7 days of vaccination per 10 million doses (138). ACIP has recommended that infants with evolving neurologic conditions should not be vaccinated with pediatric DTaP until a treatment regimen has been established and the condition has stabilized (1). Concerns about vaccinating adolescents with progressive or uncontrolled underlying neurologic disease must be weighed against the potential for morbidity from pertussis. Adolescents with severe neurologic conditions might be at risk for severe pertussis (48; CDC, unpublished data, 2005). ACIP does not consider a history of well-controlled seizures in the vaccinee or a family history of seizures (febrile or afebrile) or other neurologic disorder to be a contraindication or precaution to vaccination with pertussis components (1). ACIP has recommended that vaccine providers and parents evaluate the risks for and benefits of administering subsequent doses of vaccines with pertussis components to young children who experienced these events after pediatric DTP/DTaP: temperature >105° F (>40.5° C) within 48 hours after pediatric DTP/DTaP, not attributable to another cause; collapse or shock-like state (hypotonic hyporesponsive episode) within 48 hours after pediatric DTP/DTaP; persistent crying lasting >3 hours, occurring within 48 hours after pediatric DTP/DTaP; or convulsions with or without fever, occurring within 3 days after pediatric DTP/DTaP. All of these events were documented more frequently following whole cell pertussis vaccines than they have been following acellular vaccines (1,139--142). VAERS data have documented decreased reports of fever and other systemic events in infants and children following pediatric DTaP in comparison with pediatric DTP (143). These events either do not occur in adolescents or are of less clinical concern in this age group than in infants and children (e.g., febrile seizures and hypotonic-hyporesponsive episodes). Taken together, this information supports administering Tdap to adolescents with a history of the events listed above after pediatric DTaP/DTP. Tetanus Toxoid--Containing Vaccines ACIP has recommended that Guillain-Barré syndrome occurring <6 weeks after receipt of a tetanus toxoid--containing vaccine is a precaution for subsequent tetanus toxoid--containing vaccines (91). IOM concluded that evidence favored acceptance of a causal relation between tetanus toxoid--containing vaccines and Guillain-Barré syndrome (123). Evidence is based primarily on a well-documented case report (123,144). However, a subsequent analysis of active surveillance data in both adult and pediatric populations failed to demonstrate an association between receipt of a tetanus toxoid--containing vaccine and onset of Guillain-Barré syndrome within 6 weeks following vaccination (145). IOM also has concluded that evidence from case reports and uncontrolled studies involving tetanus toxoid--containing vaccines favored a causal relation between tetanus toxoid--containing vaccines and brachial neuritis. Brachial neuritis is considered to be a compensable event through the Vaccine Injury Compensation Program (123). However, ACIP does not consider a history of brachial neuritis to be a precaution or contraindication for administration of tetanus toxoid--containing vaccines; brachial neuritis is usually self-limited (91,143,146). Considerations for Vaccinating Pregnant Adolescents with Td or TdapACIP has recommended Td routinely for pregnant women who received the last tetanus toxoid--containing vaccine >10 years earlier. This recommendation is primarily to prevent neonatal tetanus (24,31). In women adequately vaccinated against tetanus, passive transfer of antibodies across the placenta during pregnancy protects their newborns from neonatal tetanus (147--149). During 1999, a global maternal and neonatal tetanus elimination goal was recommended by the WHO, the United Nations Children's Fund, and the United Nations Population Fund (150). As with tetanus, antibodies to pertussis antigens are passively transferred during pregnancy (151,152); however, serologic correlates of protection against pertussis are not known (88). Whether or not passive transfer of maternal antibodies to pertussis antigens protects neonates against pertussis is also unclear (88,153). All licensed Td and Tdap vaccines are categorized as Pregnancy Category C agents by FDA.¶¶ Pregnant women were excluded from prelicensure trials, and no animal reproduction studies have been conducted for Td or Tdap (32,33,98--101). Td has been used extensively in pregnant women worldwide, and no evidence indicates use of tetanus and diphtheria toxoids administered during pregnancy are teratogenic (24,154,155). Summary of the Rationale for Adolescent Tdap RecommendationsThe availability of Tdap, the first pertussis vaccines formulated for use in adolescents and adults in the United States, offers a new opportunity to reduce the burden of pertussis in this country. The primary objective of vaccinating adolescents with Tdap is to protect the vaccinated adolescent against pertussis while maintaining the standard of care for protection against tetanus and diphtheria. A secondary objective of adolescent Tdap vaccination is to reduce the reservoir of pertussis within the U.S. population at large and potentially reduce the incidence of pertussis in other age groups, including infants who have the highest risk for complications from pertussis (16,48). The extent to which the secondary objective can be achieved through adolescent vaccination is unknown. The decision to recommend routine Tdap vaccination for adolescents is based on evidence regarding the burden of pertussis among adolescents; negative effects of pertussis outbreaks involving adolescents on the community and the public health system; studies suggesting use of Tdap among adolescents will likely be safe, effective, and economical; and the established infrastructure for adolescent vaccination (30,32,33,52--54,68). To protect against pertussis, Tdap will be introduced into an adolescent vaccination schedule that already includes two other tetanus and/or diphtheria toxoid--containing vaccines, Td, and MCV4 (23,130). Frequent doses of tetanus and diphtheria toxoid--containing vaccines can be associated with increased local and systemic reactogenicity (108,109). ACIP has considered issues related to spacing and administration sequence of these three vaccines to develop recommendations for Tdap use in adolescents. Recommendations for Use of Tdap and Td Among AdolescentsThe following sections present recommendations for use of Tdap and Td among adolescents aged 11--18 years and include routine Tdap vaccination, contraindications and precautions, and special situations (Appendix C). 1. Routine Tdap Vaccination1-A. Recommendations for Use (Table 12)
1-B. Dosage and Administration
1-C. Simultaneous Vaccination with Tdap and Other Vaccines
1-D. Interchangeable Use of Tdap Vaccines
1-E. Preventing Adverse Events
1-F. Record Keeping
2. Contraindications and Precautions for Use of Tdap and Td Among Adolescents Aged 11--18 Years2-A. Contraindications
2-B. Precautions and Reasons to Defer Tdap and/or Td
2-C. Not Contraindications or Precautions
3. Special Situations for Use of Tdap and Td3-A. General Principles
3-B. Nonsimultaneous Vaccination with Tdap and Other Vaccines, Including MCV4
3-C. Pertussis Outbreaks and Other Settings with Increased Risk for Pertussis or its Complications
3-D. Lack of Availability of Tdap or MCV4
3-E. Tetanus Prophylaxis in Wound Management
3-F. History of Pertussis
3-G. Adolescents with History of Incomplete Pertussis Vaccination (Received Pediatric DT or Td Instead of Pediatric DTP/DTaP)
3-H. Adolescents with History of Incomplete Pediatric DTP/DTaP/DT or Td Vaccination
3-I. Children Aged 7--10 Years with Incomplete Pediatric DTP/DTaP Vaccination History
3-J. Inadvertent Administration of Tdap or Pediatric DTaP
3-K. Vaccination during Pregnancy
3-L. Post-Partum Vaccination
3-M. Older Adolescents and Adults Aged >18 Years
Reporting of Adverse Events After VaccinationAs with any newly licensed vaccine, surveillance for rare adverse events associated with administration of Tdap is important for assessing its safety in large-scale use. The National Childhood Vaccine Injury Act of 1986 requires health-care providers to report specific adverse events that follow tetanus, diphtheria, or pertussis vaccination (http://vaers.hhs.gov/reportable.htm). All clinically significant adverse events should be reported to VAERS, even if causal relation to vaccination is not certain. VAERS reporting forms and information are available electronically at http://www.vaers.hhs.gov/ or by telephone, (800) 822-7967. Web-based reporting is available and providers are encouraged to report electronically at https://secure.vaers.org/VaersDataEntryintro.htm to promote better timeliness and quality of safety data. Safety surveillance for adolescent Tdap, MCV4, and other vaccines is being conducted on an ongoing basis in cooperation with FDA. Previously published safety data for Td and for tetravalent meningococcal polyscaccharide vaccine will provide some of the basis for comparison with postlicensure safety surveillance for Tdap and MCV4, respectively (130,166). Vaccine Injury CompensationThe National Vaccine Injury Compensation Program (VICP), established by the National Childhood Vaccine Injury Act of 1986, is a system under which compensation can be paid on behalf of a person thought to have been injured or to have died as a result of receiving a vaccine covered by the program. Anyone receiving a covered vaccine, regardless of age, can file a petition under VICP. The program is intended as an alternative to civil litigation under the traditional tort system because negligence need not be proven. The Act establishes 1) a Vaccine Injury Table that lists the vaccines covered by the program; 2) the injuries, disabilities, and conditions (including death) for which compensation might be paid without proof of causation; and 3) the period after vaccination during which the first symptom or substantial aggravation of the injury must appear. Persons might be compensated for an injury listed in the table or one that can be demonstrated to result from administration of a listed vaccine. All tetanus toxoid--containing vaccines and vaccines with pertussis components (e.g., Tdap, Td, and pediatric DTaP) are covered under the Act. Additional information about the program is available at http://www.hrsa.gov/osp/vicp or by calling 1-800-338-2382. Areas for Future Research Related to Tdap and AdolescentsWith recent licensure and introduction of Tdap for adolescents, close monitoring of pertussis disease trends and vaccine safety will be high priorities for public health organizations and health-care providers. Active surveillance sites in Massachusetts and Minnesota, supported by CDC, are being established to provide additional data on the burden of pertussis in adolescents and other age groups and the impact of adolescent Tdap vaccination recommendations. Postlicensure studies and surveillance activities are planned or under way to evaluate
Research is needed to evaluate and define
AcknowledgementsThis report was prepared in collaboration with the Advisory Committee on Immunization Practices Pertussis Working Group. We acknowledge our U.S. Food and Drug Administration colleagues, Theresa Finn, PhD, and Ann T. Schwartz, MD, for their review of the Tdap product information and our Massachusetts Department of Public Health colleagues, Susan M. Lett, MD, Arquimedes Areche, MPH, Nancy Harrington, Kirsten Buckley, MPH, Jill Northrup, MPH, Linda Han, MD, Dara Spatz Friedman, PhD, and Stephanie Schauer, PhD, for providing data from Massachusetts used in this report. We also acknowledge the contributions of the following consultants who provided technical expertise used in the report: William L. Atkinson, MD, Jeffery P. Davis, MD, Michael D. Decker, MD, Leonard R. Friedland, MD, Daniel B. Fishbein, MD, Scott A. Halperin, MD, Kashif Iqbal, MPH, Tanya Johnson, MPH, Grace M. Lee, MD, Lucia Lee, MD, Monika Naus, MD, Ismael R. Oretga-Sanchez, PhD, Larry K. Pickering, MD, Renee Renfus, MD, Nancy Rosenstein, MD, Joel Ward, MD, and Donna Weaver, MN. We also recognize the contributions of many other consultants from academia, state health departments, federal agencies, and industry who are not listed individually in this document.
* Incidence throughout this report was calculated using confirmed and probable cases reported to the NNDSS and population estimates from U.S. Census Bureau, available at http://www.census.gov. † Booster response: In persons with prevaccination antibody concentration <0.1 IU/mL, postvaccination antibody concentration >0.4 IU/mL. In persons with prevaccination antibody concentration >0.1 IU/mL, an increase of at least four times the prevaccination antibody concentration. § Booster responses: In initially seronegative persons (<5 EU/mL [ELISA units per milliliter], postvaccination antibody concentrations >20 EU/mL. In initially seropositive persons with prevaccination antibody concentrations >5 EU/mL and <20 EU/mL, an increase of at least four times the prevaccination antibody concentration. In initially seropositive persons with a prevaccination antibody concentration of >20 EU/mL, an increase at least two times the prevaccination antibody concentration. ¶ Booster response was defined as a fourfold rise in antibody concentration, if the prevaccination antibody concentration was equal to or below the cut-off value, and a two-fold rise in antibody concentration if the prevaccination antibody concentration was above the cut-off value. The cut-off value for tetanus was 2.7 IU/mL. The cut-off value for diphtheria was 2.56 IU/mL. ** A booster response for each antigen was defined as a fourfold rise in antibody concentration, if the prevaccination antibody concentration was equal to or below the cut-off value, and a twofold rise in antibody concentration if the prevaccination antibody concentration was above the cut-off value. The cut-off values for pertussis antigens were 85 EU/mL for pertussis toxin, 170 EU/mL for filamentous haemagglutinin, 115 EU/mL for pertactin, and 285 EU/mL for fimbriae. †† An antihepatitis B surface antigen level >10 mIU/mL was considered seroprotective. §§ One month after vaccination, the antidiphtheria GMCs were ADACEL™ 8.5 IU/mL (95% confidence interval [CI] =7.6--9.5) compared with Tdsp 7.1 IU/mL (95% CI = 6.4--7.8) and BOOSTRIX® 7.4 IU/mL (95% CI = 7.1--7.6) compared with TdMPHBL14.0 IU/mL (95% CI = 13.2--14.9); the antidiphtheria seroprotective and booster response rates in the adolescents vaccinated with Tdap (ADACEL™ and BOOSTRIX®) were noninferior to the those vaccinated with Td (see sections on Immunogenicity and Serologic Bridge to Pertussis Efficacy). ¶¶ U.S. Food and Drug Administration Pregnancy Category C: Animal studies have documented an adverse effect and no adequate and well-controlled studies in pregnant women have been conducted or no animal studies and no adequate and well-controlled studies in pregnant women have been conducted *** Five doses of pediatric DTP/DTaP before the seventh birthday; if the fourth dose was administered on or after the fourth birthday, the fifth dose is not needed. ††† These conditions are precautions for use of Tdap among adolescents but are contraindications for use of pediatric DTaP among infants and children. §§§ Five doses of pediatric DTP/DTaP/DT before the seventh birthday; if the fourth dose was administered on or after the fourth birthday, the fifth dose is not needed. Children who began the tetanus and diphtheria vaccination series at age >7 years required 3 doses of Td to complete the primary series. ¶¶¶ A single dose of BOOSTRIX® Tdap is licensed for persons aged 10 years and can be used instead of Td for one of the doses in children aged 10 years; if BOOSTRIX® is administered early to a child aged 10 years, the dose counts as the adolescent Tdap dose usually administered at age 11--12 years. **** Provisional ACIP recommendations are available at http://www.cdc.gov/nip/recs/provisional_recs/default.htm; final ACIP recommendations are available at http://www.cdc.gov/nip/publications/acip-list.htm.
References
Table 1 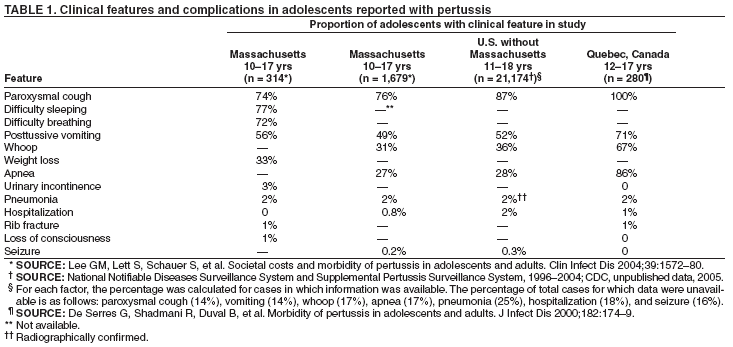 Return to top. Figure 1  Return to top. Table 2  Return to top. Figure 2  Return to top. Table 3  Return to top. Figure 3 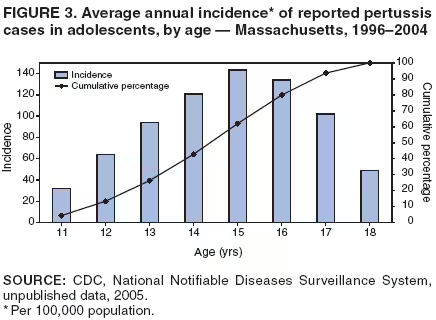 Return to top. Table 4 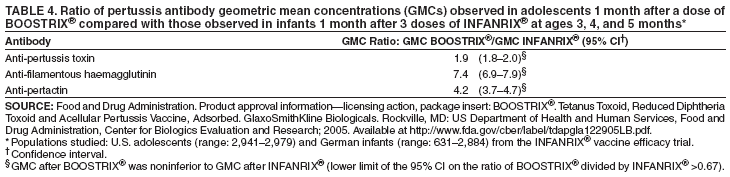 Return to top. Table 5  Return to top. Table 6  Return to top. Table 7 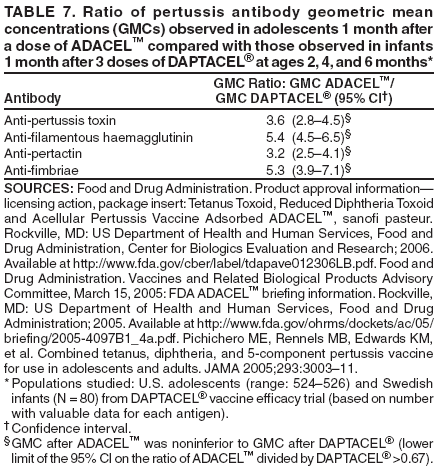 Return to top. Table 8 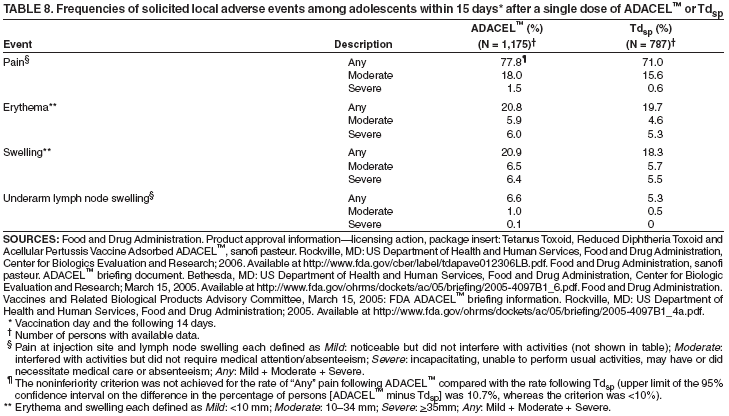 Return to top. Table 9 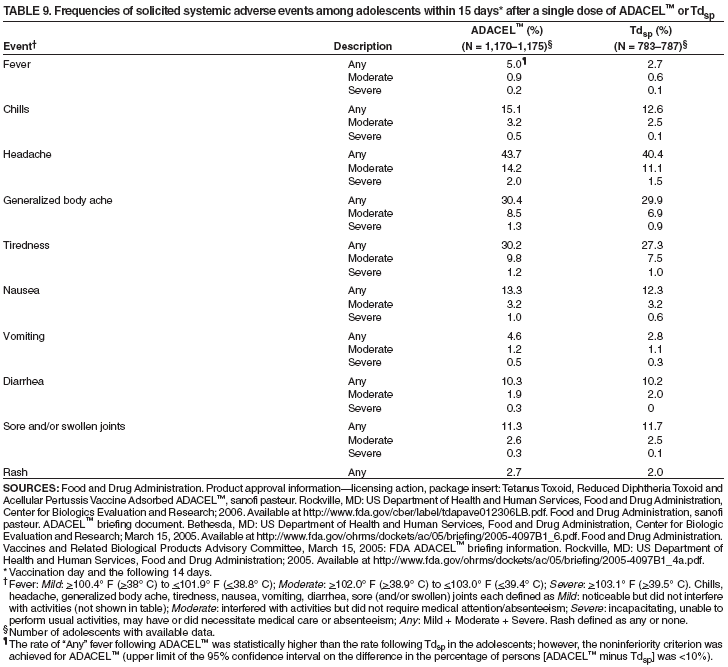 Return to top. Table 10  Return to top. Table 11 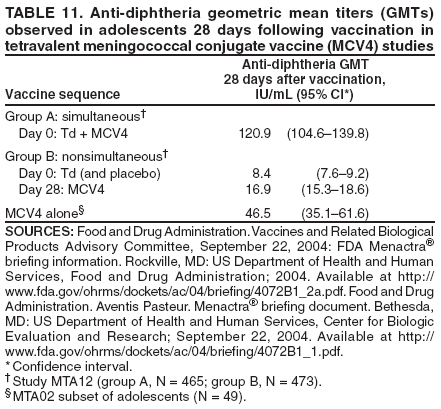 Return to top. Table 12  Return to top. Table 13 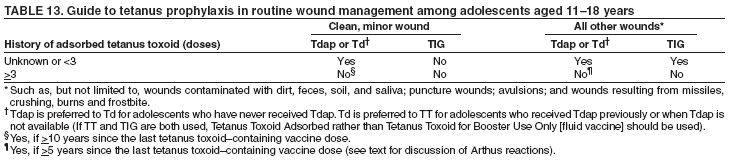 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/9/2006 |
|||||||||
|